|
Research Ideas and Outcomes :
Grant Proposal
|
|
Corresponding author: Helen R P Phillips (helen.phillips@smu.ca)
Received: 30 May 2022 | Published: 31 Aug 2022
© 2022 Helen Phillips, Erin Cameron, Nico Eisenhauer
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Phillips HRP, Cameron EK, Eisenhauer N (2022) Illuminating biodiversity changes in the ‘Black Box’. Research Ideas and Outcomes 8: e87143. https://doi.org/10.3897/rio.8.e87143
|
|
Abstract
Soil is often described as a ‘black box’, as surprisingly little is known about the high levels of biodiversity that reside there. For aboveground organisms, we have good knowledge of the distribution of the species and how they might change under future human impacts. Yet despite the fact that soil organisms provide a wide variety of ecosystem functions, we have very limited knowledge of their distribution and how their diversity might change in the future. In order to create accurate and generalisable models of biodiversity, the underlying data need to be representative of the entire globe. Yet even with our recently compiled global earthworm dataset of over 11000 sites, there are gaps across large regions. These gaps are consistent across many other datasets of both above- and belowground diversity. In order to fill the gaps we propose a sampling network (SoilFaUNa), to create a comprehensive database of soil macrofauna diversity and soil functions (e.g. decomposition rates). Building on the existing dataset of earthworm diversity and early data from the SoilFaUNa project, we will investigate changes in earthworm diversity. From our current work, we know that both climate and land use are main drivers in predicting earthworm diversity, but both will change under future scenarios and may alter ecosystem functions. We will, using space-for-time substitution models, estimate how earthworm diversity and their functions might change in the future, modelling earthworm diversity as a function of climate, land use and soil properties and predicting based on future scenarios. Previous studies of aboveground diversity changes over time using time-series analysis have found no-net-loss in richness, but analyses have criticisms. We aim to use time-series data on earthworms to move this debate forward, by using data and statistical methods that would address the criticisms, whilst increasing our knowledge on this understudied soil group. Field experiments and micro-/mesocosm experiments have been used to investigate the link between a number of soil organisms and ecosystem functions under few environmental conditions. Meta-analyses, which can produce generalisable results can only answer questions for which there are data. Thus, we have been lacking on information on the link between the entire community of soil fauna and ecosystem functions and impact of changes to the soil fauna community across environmental contexts. Using data collected from the SoilFaUNa project, we will, for the first time, synthesise globally distributed specifically-sampled data to model how changes in the community composition of soil macrofauna (due to changes in land use, climate or soil properties) impact the ecosystem functions in the soil.
Keywords
earthworms, soil macrofauna, soil functions, ecosystem functions, global biodiversity monitoring
List of participants
Dr. George Brown, Dr. Ika Djukic, Dr. Carlos Guerra, Dr. Patrick Lavelle
State of the art and preliminary work
Loss of biodiversity
The diversity of life on Earth is declining through species extinctions (
Recent large-scale synthesis analyses have shown how local biodiversity (i.e. a measure of the ecological assemblage within a sampled plot, which can vary in size from metres to kilometres, sensu
Earthworms as a model organism for biodiversity research
Organisms that are ecosystem engineers, those that create, modify or maintain habitats (
The relatively large body size of earthworms compared to other soil taxa (
The work that we are currently undertaking will further our understanding of the current distribution of earthworm diversity and the importance of different environmental drivers in shaping the communities (
Gaps in global datasets
For decades, papers have commented on the lack of biogeographic studies for soil fauna (
However, in all of the globally assembled databases (e.g. PREDICTS:
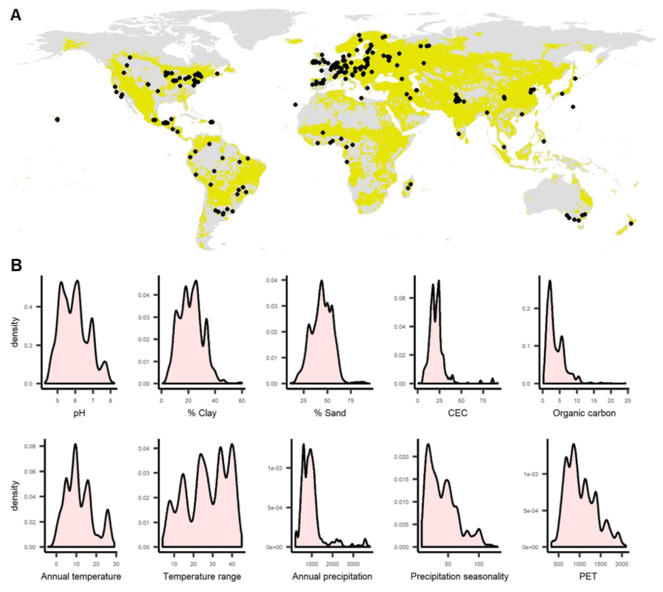
Figure 1: A) Black dots show sample locations of the 11,009 sites in the
Such gaps in global datasets could be filled by developing a global network of researchers who apply standardised protocols across sites. Previously, standardised networks of experiments have been useful in addressing typically local-scale ecological questions across a global scale (e.g. NutNet:
Projecting changes in earthworm diversity under future scenarios of change
With predicted future changes in anthropogenic pressures, such as climate change (
There are few studies that use future scenarios to project changes in local biodiversity at global scales. Those that do are often limited to only one driver of change, for example, land use (
Time series analysis
There is an ongoing debate surrounding the direction and magnitude of local biodiversity change, with some synthesis studies showing loss of local biodiversity (
Although analysis of time-series data is highly valuable for examining dynamic changes in local biodiversity, there have been criticisms of both the
The synthesis of space-for-time data is often used as more data are available, as primary datasets require only one season of fieldwork (
Both techniques have downfalls and, consequently, an ideal solution is to combine the two approaches. Using time-series data to show dynamically how local biodiversity is changing, while addressing previous criticisms by incorporating baselines and information on external anthropogenic pressures, may move this debate forward. Given the amount of earthworm data available, and the fact that a time-series analysis has not been performed on any soil taxa, earthworms are an ideal study organism with which to combine these approaches.
Loss of ecosystem services
Soil biodiversity is a key driver of many vital ecosystem functions (
As an alternative, meta-analysis can use globally distributed datasets to create more generalisable results (
Disturbances (a term used here to describe a site that differs from a ‘reference’ site, for example, a change in land use or soil properties and, therefore, not necessarily due to human impacts) are likely to change the composition of communities, but exactly how composition will change may vary. Groups of taxa may be lost or changes in abundance or biomass may occur (
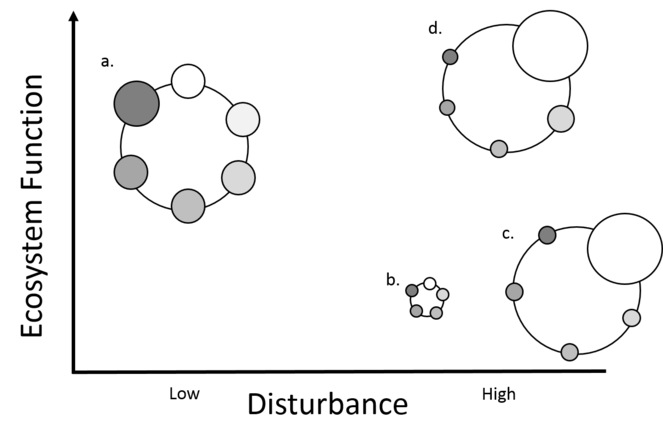
Figure 2: The hypothetical changes in community composition as a result of changes in the disturbance across sites and their potential impact on ecosystem function. The X-axis shows two states of disturbance, ‘low’ (a baseline/reference) and ‘high’ (a change in the disturbance, such as a non-natural land use). Y-axis shows the potential amount or rate of an unspecified ecosystem function provided by the community. Each large circle is a community, composed of multiple smaller circles of different taxa groups (e.g. earthworms, carabid beetles, millipedes etc.) The size of the smaller circles indicates abundance or biomass of that group. The community changes from the reference state (a) when the disturbance changes (b, c, d). The community could change in equal proportions (b) or in dominance structure (c and d). The changes could result in a reduction in the ecosystem function that the community provides (b and c) or the ecosystem function could be maintained (d), especially if the dominant group is the main contributor to the ecosystem function measured.
Despite its importance, very little is known about the biodiversity in the ‘black box’ (
Project-related publications
Articles published by outlets with scientific quality assurance, book publications and works accepted for publication, but not yet published.
Phillips HRP, Guerra CA,….and Eisenhauer N (2019) Global distribution of earthworm diversity. Science 366: 480–485
Phillips HRP, Cameron EK, Ferlian O, Türke M, Winter M, Eisenhauer N (2017) Red list of a black box. Nature Ecology and Evolution 1: article no 0103.
Cameron E, Martins IS, Lavelle P,… Phillips HRP,… Eisenhauer N (2018) Global gaps in soil biodiversity data. Nature Ecology and Evolution 2(7): 1042—1043.
Phillips HRP, Newbold T, Purvis A (2017) Land-use effects on local biodiversity in tropical forests vary between continents. Biodiversity and Conservation 26 :2251-2270.
Newbold T, Hudson LN, … Phillips HRP, …and Purvis A (2016) Has land use pushed terrestrial biodiversity beyond the planetary boundary? A global assessment. Science 353: 288-291.
Newbold T, Hudson LN, … Phillips HRP, …and Purvis A (2015) Global effects of land use on local terrestrial biodiversity. Nature 520: 45-50.
Eisenhauer N, Dobies T, Cesarz S, Hobbie SE, Meyer RJ, Worm K, Reich PB (2013) Plant diversity effects on soil food webs are stronger than those of elevated CO2 and N deposition in a long-term grassland experiment. Proceedings of the National Academy of Sciences USA 110: 6889-6894.
Isbell F, Craven D, Connolly J, et al. and Eisenhauer N (2015) Biodiversity increases the resistance of ecosystem productivity to climate extremes. Nature 526: 574-577.
Reich PB, Tilman D, Isbell F, Mueller K, Hobbie SE, Flynn DFB and Eisenhauer N (2012) Impacts of biodiversity loss escalate through time as redundancy fades. Science 336: 589-592.
Schwarz B, Barnes AD, Thakur MP, Brose U, Ciobanu M, Reich PB, Rich RL, Rosenbaum B, Stefanski A and Eisenhauer N (2017) Warming alters energetic structure and function but not resilience of soil food webs. Nature Climate Change 7: 895-900.
Objectives, concept and approach
Objectives
Although there have been many small-scale studies on changes in soil biodiversity, there has been little progress in creating large-scale, generalisable results. This project aims to investigate changes in soil biodiversity (with a focus on earthworms, Order: Crassiclitellata), across large spatial scales using synthesis analyses. In addition, the project will also link soil biodiversity to soil functions and investigate how any changes in biodiversity might impact the ecosystem functions upon which we rely. Using previously collated data, as well as data collected specifically for this project and appropriate statistical methods that deal with the complexities of the data and the ecological questions, we will advance the field of global soil biodiversity.
Although soil biodiversity data are available across the globe the distribution is poor, especially within certain regions, and heavily biased towards certain realms and environmental conditions (Fig.
WP2, 3 and 4 aim to further both our knowledge of the changes in soil biodiversity, particularly earthworms, whilst also furthering key, timely and highly relevant questions in the field of biodiversity change and ecosystem function. Previous synthesis analyses have shown how biodiversity is predicted to respond under future scenarios of change and have investigated whether biodiversity has been changing over time. However, these questions have been studied using datasets primarily composed of aboveground organisms. WP2 and 3 will modify the previously-used methods, to answer these questions in relation to earthworms. In addition, by modifying the methods previously used, we aim to further the field by addressing key questions that have not been addressed previously, such as whether local earthworm biodiversity is changing over time.
Providing this research is crucial, given that the understudied soil biodiversity is relied upon heavily for many ecosystem functions. In WP2, we aim to show how changes in earthworm diversity (as predicted based on future scenarios of global change) might affect the ecosystem functions that they provide. While, in WP4, we will further extend the biodiversity-ecosystem function field, by researching how multiple ecosystem functions provided by the soil might change when the soil community is altered, using specifically-collected datasets from non-manipulated biodiversity measurements in a globally-distributed network.
This project will be led by Dr Helen Phillips in the Experimental Interaction Ecology group of Prof. Nico Eisenhauer, at the University of Leipzig and the German Centre for Integrative Biodiversity Research (iDiv) Halle-Jena-Leipzig. Dr Helen Phillips was the lead post-doc (as part of a working group led by Prof. Nico Eisenhauer) collating data of earthworm diversity measures. This dataset now allows for a number of novel analyses.
Anticipated total duration of the project
Current funding: none.
Duration: 3 years
Work programme including proposed research methods
WP1 - Monitoring Scheme
Objectives
The number of research papers using synthesis approaches (i.e. collating raw data from previously published papers to conduct new analyses) to address patterns in biodiversity and questions on biodiversity change has recently increased (
We will create a network of researchers (the Soil biodiversity and Soil Function Network- SoilFaUNa) to collect soil biodiversity and soil functions data from regions that are typically understudied. The protocols used will be standardised and simple, where possible being based on protocols developed and agreed upon by soil experts within iSBio (https://home.uni-leipzig.de/idiv/isbio/;
Methods
At the start of the project, we will re-contact the ~ 200 earthworm researchers we have previously worked with. We will ask researchers who have collected earthworm data in the past if they can resample the earthworm communities of their previously-sampled sites using their original methodology, in order to obtain more time-series datasets (suitable for analysis in WP3). They will also be informed about our new standardised sampling protocol (see below) and will be invited to start new sampling campaigns to collect data suitable for WP2 and WP4 (soil fauna and functions). We will also locate new soil ecologists to collaborate with, by searching through universities in target regions, for example across the tropics (i.e. Indonesia), central Africa (i.e. Tanzania), as well as boreal Asia (i.e. northern China). We believe that our advertising papers will also prompt soil ecologists to contact us. Nico Eisenhauer has successfully tested this approach recently in a global collection of soil microbial biomass data (
To standardise sampling and assist researchers who may have limited experience in sampling soil fauna, an easily reproducible protocol has been drafted and will be distributed to all collaborating researchers (Suppl. material
Collaborating researchers for the 200 new sites will be asked to sample earthworms, other soil macrofauna and soil functions at more than one site at least twice (at months 3 and 12 when teabags are collected; see below). Within each collaborating researcher’s sampling campaign, sites must be distributed across a range of current environmental conditions (e.g. land use, habitat cover, soil, climate), in the hope of increasing the environmental coverage of the database as a whole (Fig.
Soil functions will also be measured at each site. To measure the belowground decomposition rate, the Tea Bag Index method will be used, which estimates decomposition rates by calculating weight differences in tea bags over time (see
All collaborating researchers will be sent a standardised data template, into which they will enter meta-data and biodiversity data and soil function data from each site sampled. This will ensure that all necessary data are collected and are stored in a standardised form, thereby decreasing the time needed to process it ready for analysis. In addition to our own global initiatives (e.g.
Mustard powder, teabags and containers for soil samples will be sent to all collaborating researchers, with the remaining costs of all fieldwork being covered by the collaborating researcher; however, protocols have been designed to use inexpensive methods/equipment. Collaborating researchers will be responsible for acquiring any permits needed for collecting and moving soil samples out of their country. We will obtain any permits that are needed to move soil samples into Germany (such as done in previous international, collaborative projects, e.g.
Data that are analysed at iDiv would be shared with the original collaborating researcher within the timeframe of the project. A subsample of 2 g of all soil samples would be stored at -80°C long term (at iDiv) to enable genetic analyses to be undertaken in the future. In addition, all biodiversity data would be entered into the Global Soil Biodiversity Database. We will ask all collaborating researchers to store biological samples for five years post sampling in 95% alcohol (see sampling protocol in Suppl. material
2.3.2 WP2 - Projecting changes in earthworm diversity under future scenarios of change
Objectives
From previous work, we are beginning to understand the large-scale spatial patterns of earthworm diversity in relation to current environmental conditions, such as soil properties, land use and climate (
We propose, using previously collected data (11,009 sites,
Methods
For this WP, we will use previously-collected data (Fig.
For datasets to be suitable for this analysis, they will need to contain earthworm data from across more than two sites where current environmental conditions (e.g. land use, climate, soil properties) vary. The exact position of each site would also need to be known (from either GPS coordinates or by digitising and geolocation of available maps).
In order to create biodiversity projections using scenarios of future change (e.g.
2.3.3 WP3 - Time-series analysis
Objectives
Debate has continued over whether local biodiversity is declining (
In this WP3, we will use the time-series analysis approach similar to that used by
Methods
Suitable datasets collected previously (
The earthworm samples from each sampled time will be temporally matched to both soil properties (measured either by researchers at the same time as sampling or from global data layers, such as SoilGrids;
Data will be analysed using a linear mixed effects model framework using ‘lme4’ in R. The random effect structure will account for differences between different datasets, such as sampling methodology, researcher error/biases, as well as differences from study location. Based on suggestions in
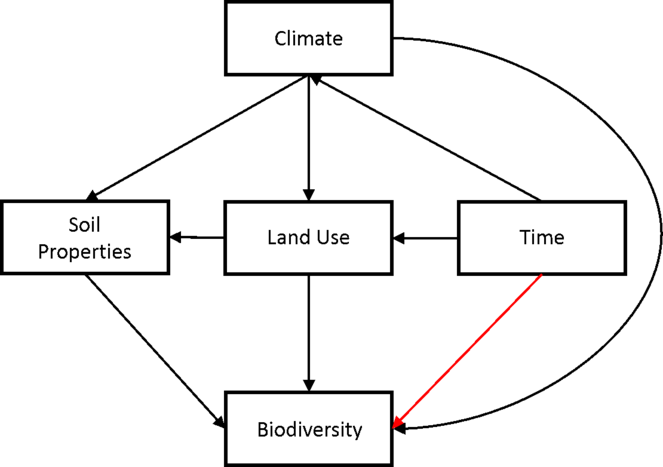
We will also investigate alternative modelling approaches. One potential alternative will be the use of structural equation models (SEMs;
Figure 3: Structural Equation “meta-“ model (
2.3.4 WP4 - Ecosystem functions
Objectives
Soil biodiversity is important for ecosystem functions and, consequently, changes in soil biodiversity may impact functions (e.g.
In this WP, we will use specifically collected data on the soil community and ecosystem functions (from WP1) to investigate how soil communities change with disturbance and how ecosystem functions change as a result. We will investigate the following hypotheses (see Fig. 2): In a disturbed site, relative to an undisturbed site, all soil taxa groups are negatively impacted (decrease in community abundance) and ecosystem function is reduced (WP4-H1). Alternatively, not all soil taxa groups in a disturbed site will be negatively impacted (change in community composition) and ecosystem function is changed (WP4-H2). A third option is that in a disturbed site not all soil taxa groups are negatively impacted (change in community composition) but ecosystem function is maintained (WP4-H3).
Methods
Data will be collated on soil fauna and soil functions through the establishment of the global monitoring network in WP1. These data, collected using a standardised sampling protocol from sites distributed across the globe, will be analysed as part of this WP. A literature search will be performed to obtain recent published studies that meet all criteria. In addition, we are also aware of one large dataset (Biodiversity Exploratories) that has collected suitable data from across 150 sites in Germany. We will ask for access to this dataset to add to the analysis.
Suitable datasets will include data from two or more sites, which vary in their environmental conditions (e.g. land use, habitat cover, soil properties, climate). Each dataset will contain the sampled diversity (biomass and abundance) of soil macrofauna at the order-level (e.g. earthworms, beetles, centipedes/millipedes), as well as the biomass/abundance/diversity of the earthworm community. In addition, soil functions (decomposition, soil pH, aggregate stability, microbial C, microbial respiration, metabolic quotient (qO2)) at each site will also have been measured (discussed in WP1). As the coordinates of each site will be known, external data layers (such as SoilGrids and CHELSA climate data) can also be included in the analysis.
Although all the data will be standardised across the datasets, mixed effect models will be tested in case differences between datasets that may have arisen due to differences amongst multiple data collectors need to be accounted for. Models will be constructed for each ecosystem function. Predictor variables will include environmental variables that were collected in situ, such as land use and habitat cover, as well as those acquired from global layers that may explain additional variance within the data, such as altitude.
As little is known about how the diversity and composition of soil communities will change across a range of environmental conditions, such as different land uses, habitat covers and climates, we will first investigate this using the collated dataset. Diversity measures of the soil community, such as biomass, abundance and composition (discussed below), will be used as response variables in the models, with information on the environmental conditions at each site used as predictor variables.
To test whether changes in composition affect ecosystem functions, models will also contain the community composition of the soil fauna at the site as a predictor variable. The suitability of community composition metrics for the analysis will be investigated, to ensure that the abundance or biomass of each taxonomic order is clearly captured. For example, Simpson’s Evenness (
The ecosystem functions that we are measuring may not be strongly impacted by all of the macrofauna groups sampled. Earthworms are known to impact some of the ecosystem functions measured, for example, decomposition, microbial activity, potentially more so than the other soil fauna groups that may be sampled. Therefore, models will also be created using earthworm diversity and the composition of the earthworm functional groups as the predictor variables (along with predictor variables mentioned previously) of the ecosystem functions. Further investigations into the effect of different soil fauna orders can then be done post hoc if needed.
Deliverables
WP1: At least one paper will be written to highlight and promote the creation of this monitoring network. In order to increase the participation in the under-represented countries, the paper will be submitted to a continent-specific, peer-reviewed journal. A more general paper, detailing the creation of the network and the subsequent database, will also be submitted on completion of the project to an international, peer-reviewed journal.
WP2: This synthesis analysis will allow us to write at least one paper in an international, peer-reviewed journal on the changes in earthworm communities as a response to human impacts.
WP3: This synthesis analysis will result in at least one paper in an international, peer-reviewed journal showing how earthworm communities are changing over time, adding to the existing debate on whether local diversity is changing over time.
WP4: This work package will result in at least one paper in an international, peer-reviewed journal. The paper will focus on using a collaborative network of researchers to address how soil biodiversity is changing in response to disturbance and the effects on the ecosystem functions provided. All collaborating researchers will be invited to be an author on the paper.
Data handling
All data will be made publicly available with the respective publication using common data repositories, such as Dryad (http://datadryad.org/) and Pangaea (https://www.pangaea.de/), and assigned a DOI. Further, all data will be submitted to the Global Soil Biodiversity Database.
Additional information
Protocols for WP1 (See Supplementary Materials)
Project requirements
Employment status information
Phillips, Helen, Post-Doctoral Researcher, (currently based at the German Centre for Integrative Biodiversity Research (iDiv) Halle-Jena-Leipzig, Deutscher Platz 5e, 04103 Leipzig, Germany, (DFG FZT 118))
Eisenhauer, Nico, Full Professor (W3), permanent
First-time proposal data
Phillips, Helen
German Centre for Integrative Biodiversity Research (iDiv) Halle-Jena-Leipzig, Deutscher Platz 5e, 04103 Leipzig, Germany
Composition of the project group
Dr. Simone Cesarz, permanent post-doc in the Eisenhauer lab, specialist in soil chemical analyses. She will provide additional support and expertise for laboratory work. Anja Zeuner, technician within the Eisenhauer lab. She will conduct some of the laboratory work and provide assistance in the lab, if needed. Svenja Haenzel, Foreign Language Secretary in the Eisenhauer lab, will support the project by helping with contracting the student helper and with providing the necessary shipping documents and permits.
Cooperation with other researchers
Researchers with whom you have agreed to cooperate on this project
Dr. Ika Djukic, leads the global TeaComposition project (
Researchers with whom you have collaborated scientifically within the past three years
Prof. Andy Purvis and all members of the PREDICTS project (http://www.predicts.org.uk/pages/team.html). Dr. Nicholas Fisichelli, Dr. Lee Frelich, Prof. Sarah Hobbie, Dr. Forest Isbell, Prof. Dr. Edward Johnson, Dr. Pete Manning, Prof. Peter Reich, Prof. Matthias Rillig, Prof. Jacques Roy, Prof. David Tilman, Prof. Wim van der Putten, Prof. Alexandra Wright, all PIs of iDiv (https://www.idiv.de/groups_and_people/members.html) and the Jena Experiment (http://www.the-jena-experiment.de/Members.html) and all editors of Pedobiologia – Journal of Soil Ecology.
Scientific equipment
A laboratory, with all necessary equipment for WP1, is available at iDiv (within the Experimental Interaction Ecology group led by Prof. Nico Eisenhauer). In addition, iDiv provides a high-performance computer cluster (HPC) and highly skilled IT support staff, that would be available should the analysis in WP2-4 require additional computational power. iDiv would provide the perfect infrastructure for the present project and no further equipment is requested.
References
- Effects of the fungicide pyrimethanil on biofilm and organic matter processing in outdoor lentic mesocosms.Ecotoxicology25(1):121‑131. https://doi.org/10.1007/s10646-015-1574-x
- Languages Are Still a Major Barrier to Global Science.PLOS Biology14(12). https://doi.org/10.1371/journal.pbio.2000933
- Tropical Soil Biology and Fertility: A handbook of methods.Tropical Soil Biology and Fertility: A handbook of methods2 Ed.:88‑91. https://doi.org/10.1017/S0014479700018354
- Soil bacterial diversity in degraded and restored lands of Northeast Brazil.Antonie van Leeuwenhoek, International Journal of General and Molecular Microbiology106(5):891‑899. https://doi.org/10.1007/s10482-014-0258-5
- Belowground biodiversity and ecosystem functioning.Nature 515505(7528):505‑511. https://doi.org/10.1038/nature13855
- Fitting Linear Mixed-Effects Models Using lme4.Journal of Statistical Software67(1):1‑48. https://doi.org/10.18637/jss.v067.i01
- A review of earthworm impact on soil function and ecosystem services.European Journal of Soil Science64(2):161‑182. https://doi.org/10.1111/ejss.12025
- The geography of biodiversity change in marine and terrestrial assemblages.Science366(6463):339‑345. https://doi.org/10.1126/science.aaw1620
- Finding generality in ecology: A model for globally distributed experiments.Methods in Ecology and Evolution5(1):65‑73. https://doi.org/10.1111/2041-210X.12125
- A decade of insights into grassland ecosystem responses to global environmental change.Nature Ecology & Evolution1(5). https://doi.org/10.1038/s41559-017-0118
- Earthworms, water infiltration and soil stability: Some new assessments.Soil Biology and Biochemistry29(3-4):441‑452. https://doi.org/10.1016/S0038-0717(96)00272-6
- Strategies lombriciennes.Ecological Bulletins(25)122‑132.
- Impacts of Soil Faunal Community Composition on Model Grassland Ecosystems.Science298:615‑618. https://doi.org/10.1126/science.1075805
- How do earthworms affect microfloral and faunal community diversity?Plant and Soil170(1):209‑231. https://doi.org/10.1007/BF02183068
- Biodiversity and ecosystem functioning in soil.Ambio: A Journal of the Human Environment26(8):563‑570. https://doi.org/10.2307/1313535
- The Edaphobase project of GBIF-Germany-A new online soil-zoological data warehouse.Applied Soil Ecology83:3‑12. https://doi.org/10.1016/j.apsoil.2014.03.021
- Global biodiversity: indicators of recent declines.Science328(5982):1164‑1168. https://doi.org/10.1126/science.1187512
- Global gaps in soil biodiversity data.Nature Ecology & Evolutionin press:6‑7. https://doi.org/10.1038/s41559-018-0573-8
- Earthworm databases and ecological theory: Synthesis of current initiatives and main research directions.Applied Soil Ecology104(August):85‑90. https://doi.org/10.1016/j.apsoil.2015.11.012
- Overlooked local biodiversity loss.Science344(6188).
- Is local biodiversity declining or not? A summary of the debate over analysis of species richness time trends.Biological Conservation219:175‑183. https://doi.org/10.1016/j.biocon.2017.12.021
- Biodiversity loss and its impact on humanity.Nature486(7401):59‑67. https://doi.org/10.1038/nature11148
- Plant species richness sustains higher trophic levels of soil nematode communities after consecutive environmental perturbations.Oecologia184(3):715‑728. https://doi.org/10.1007/s00442-017-3893-5
- Biotic and abiotic drivers of soil microbial functions across tree diversity experiments.bioRxivhttps://doi.org/10.1101/2020.01.30.927277
- Fundamentals of Soil Ecology.Elsevier,27pp. [ISBN978-0-12-179726-3] https://doi.org/10.1016/B978-012179726-3/50009-5
- Monitoring change in vertebrate abundance: the Living Planet Index.Conservation Biology23(2):317‑327. https://doi.org/10.1111/j.1523-1739.2008.01117.x
- The unseen invaders: introduced earthworms as drivers of change in plant communities in North American forests (a meta-analysis).Global Change Biology23(3):1065‑1074. https://doi.org/10.1111/gcb.13446
- Factors affecting the abundance of earthworms in soils. In:Earthworm Ecology.22pp. [ISBN1420039717]. https://doi.org/10.1201/9781420039719.pt3
- A global atlas of the dominant bacteria found in soil.Science359(6373):320‑325. https://doi.org/10.1126/science.aap9516
- Challenges With Inferring How Land-Use Affects Terrestrial Biodiversity: Study Design, Time, Space and Synthesis - Next Generation Biomonitoring: Part 1. In:Next Generation Biomonitoring: Part 1.36pp. https://doi.org/10.1016/bs.aecr.2017.12.004
- Soil food web properties explain ecosystem services across European land use systems.PNAS110(35):14296‑14301. https://doi.org/10.1073/pnas.1305198110
- Earthworm communities in grasslands and horticultural soils.Biology and Fertility of Soils33(2):111‑117. https://doi.org/10.1007/s003740000297
- Defaunation in the Anthropocene.Science345(6195):401‑406. https://doi.org/10.1126/science.1251817
- Early stage litter decomposition across biomes.Science of Total Environment629:1369‑1394. https://doi.org/10.1016/j.scitotenv.2018.01.012
- Assemblage time series reveal biodiversity change but not systematic loss.Science344(6181):296‑299. https://doi.org/10.1126/science.1248484
- Recovery of faunal communities during tropical forest regeneration.Conservation Biology18(2):302‑309. https://doi.org/10.1111/j.1523-1739.2004.00151.x
- Global ant (Hymenoptera : Formicidae) biodiversity and biogeography – a new database and its possibilities.Myrmecological News10(September):77‑83.
- Biology and ecology of earthworms. Third edition.Springer[ISBN0412561603]
- Earthworm and belowground competition effects on plant productivity in a plant diversity gradient.Oecologia161(2):291‑301. https://doi.org/10.1007/s00442-009-1374-1
- The action of an animal ecosystem engineer: Identification of the main mechanisms of earthworm impacts on soil microarthropods.Pedobiologia53(6):343‑352. https://doi.org/10.1016/j.pedobi.2010.04.003
- From patterns to causal understanding: Structural equation modeling (SEM) in soil ecology.Pedobiologia58(2-3):65‑72. https://doi.org/10.1016/j.pedobi.2015.03.002
- Biodiversity-ecosystem function experiments reveal the mechanisms underlying the consequences of biodiversity change in real world ecosystems.Journal of Vegetation Science27:1061‑1070. https://doi.org/10.1111/jvs.12435
- Priorities for research in soil ecology.Pedobiologia63:1‑7. https://doi.org/10.1016/j.pedobi.2017.05.003
- Plant diversity maintains multiple soil functions in future environments.eLifehttps://doi.org/10.7554/eLife.41228
- Mechanisms Underlying the Relationship Between Biodiversity and Ecosystem Function.Academic Presshttps://doi.org/10.1016/S0065-2504(19)30044-3
- Recent Trends in Local-Scale Marine Biodiversity Reflect Community Structure and Human Impacts.Current Biology25(14):1938‑1943. https://doi.org/10.1016/j.cub.2015.05.030
- European Journal of Soil Biology Impact of ecologically different earthworm species on soil water characteristics.European Journal of Soil Biology45(3):207‑213. https://doi.org/10.1016/j.ejsobi.2009.01.001
- Diversity and abundance of earthworms in land use systems in central-western Colombia.Pedobiologia54(SUPPL.). https://doi.org/10.1016/j.pedobi.2011.09.016
- Effects of fungicides on decomposer communities and litter decomposition in vineyard streams.Science of the Total Environment533:40‑48. https://doi.org/10.1016/j.scitotenv.2015.06.090
- Analyzing the effects of species gain and loss on ecosystem function using the extended Price equation partition.Oikos121(2):290‑298. https://doi.org/10.1111/j.1600-0706.2011.19656.x
- Do space-for-time assessments underestimate the impacts of logging on tropical biodiversity? An Amazonian case study using dung beetles.Journal of Applied Ecology53(4):1098‑1105. https://doi.org/10.1111/1365-2664.12657
- Coordinated distributed experiments: an emerging tool for testing global hypotheses in ecology and environmental science.Frontiers in Ecology and the Environment11(3):147‑155. https://doi.org/10.1890/110279
- On the misuse of residuals in ecology: regression of residuals vs. multiple regression.Journal of Animal Ecology71(3):542‑545. https://doi.org/10.1046/j.1365-2656.2002.00618.x
- Climate change and habitat conversion favour the same species.Ecology letters19(9):1081‑1090. https://doi.org/10.1111/ele.12645
- Climate velocity and the future global redistribution of marine biodiversity.Nature Climate Change6(1):83‑88. https://doi.org/10.1038/nclimate2769
- Estimating local biodiversity change: a critique of papers claiming no net loss of local diversity.Ecology97(8):1949‑1960. https://doi.org/10.1890/15-1759.1
- Earthworm abundance and species composition in abandoned tropical croplands: comparisons of tree plantations and secondary forests.Pedobiologia40:385‑391. https://doi.org/10.1007/s10530-006-9023-7
- Structural Equation Modeling and Natural Systems.Cambridge University Presshttps://doi.org/10.1086/586991
- Guidelines for a graph-theoretic implementation of structural equation modeling.Ecosphere3(8):1‑44. https://doi.org/10.1890/ES12-00048.1
- Consequences of biodiversity loss for litter decomposition across biomes.Nature509(7499):218‑221. https://doi.org/10.1038/nature13247
- Biodiversity and Litter Decomposition in Terrestrial Ecosystems.Annual Review of Ecology, Evolution, and Systematics36(1):191‑218. https://doi.org/10.1146/annurev.ecolsys.36.112904.151932
- Biodiversity Effects on Soil Processes Explained by Interspecific Functional Dissimilarity.Science306(10):8‑10. https://doi.org/10.1126/science.1101865
- Microbial diversity-ecosystem function relationships across environmental gradients.Research Ideas and Outcomes6https://doi.org/10.3897/rio.6.e52217
- Pandora's Box Contained Bait: The Global Problem of Introduced Earthworms.Annual Review of Ecology, Evolution, and Systematics39(1):593‑613. https://doi.org/10.1146/annurev.ecolsys.39.110707.173426
- SoilGrids250m: Global gridded soil information based on machine learning.PLOS ONE12(2). https://doi.org/10.1371/journal.pone.0169748
- Consequences of dominance: a review of evenness effects on local and regional ecosystem processes.Ecology89(6):1510‑1520. https://doi.org/10.1890/07-1053.1
- Biodiversity change is uncoupled from species richness trends: Consequences for conservation and monitoring.Journal of Applied Ecology55(1):169‑184. https://doi.org/10.1111/1365-2664.12959
- A global synthesis reveals biodiversity loss as a major driver of ecosystem change.Nature486(7401):105‑108. https://doi.org/10.1038/nature11118
- The database of the PREDICTS (Projecting Responses of Ecological Diversity In Changing Terrestrial Systems) project.Ecology and Evolution7(1):145‑188. https://doi.org/10.1002/ece3.2579
- Modelling the effects of loss of soil biodiversity on ecosystem function.Global change biology8:33‑50. https://doi.org/10.1046/j.1365-2486.2002.00425.x
- Harmonization of land-use scenarios for the period 1500--2100: 600 years of global gridded annual land-use transitions, wood harvest, and resulting secondary lands.Climatic change109(1-2):117‑161. https://doi.org/10.1007/s10584-011-0153-2
- Summary for policymakers of the methodological assessment of scenarios and models of biodiversity and ecosystem services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services.Secretariat of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem
- Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change.Cambridge University Press[ISBN9789291691432] https://doi.org/10.1017/CBO9781107415324.004
- Soil quality - Sampling of soil invertebrates - Part 1: Hand-sorting and extraction of earthworms (ISO/FDIS 23611-1:2018).
- Organisms as Ecosystem Engineers.Oikos69(3). https://doi.org/10.2307/3545850
- Climatologies at high resolution for the earth’s land surface areas.Scientific Data4https://doi.org/10.1038/sdata.2017.122
- TRY -- a global database of plant traits.Global change biology17(9):2905‑2935. https://doi.org/10.1111/j.1365-2486.2011.02451.x
- Tea Bag Index: A novel approach to collect uniform decomposition data across ecosystems.Methods in Ecology and Evolution4(11):1070‑1075. https://doi.org/10.1111/2041-210X.12097
- Handbook of meta-analysis in ecology and evolution.Princeton University Press[ISBN9780691137292] https://doi.org/10.2307/j.ctt24hq6n
- Global patterns and determinants of vascular plant diversity.Proceedings of the National Academy of Sciences104(14):5925‑5930. https://doi.org/10.1073/pnas.0608361104
- Global land use change, economic globalization, and the looming land scarcity.Proceedings of the National Academy of Sciences108(9):3465‑3472. https://doi.org/10.1073/pnas.1100480108
- Soil function in a changing world: the role of invertebrate ecosystem engineers.European Journal of Soil Biology33(May 1996):159‑193.
- A test of the 'hot' mustard extraction method of sampling earthworms.Soil Biology and Biochemistry34(4):549‑552. https://doi.org/10.1016/S0038-0717(01)00211-5
- piecewiseSEM: Piecewise structural equation modelling in R for ecology, evolution, and systematics.Methods in Ecology and Evolution7(5):573‑579. https://doi.org/10.1111/2041-210X.12512
- Greenhouse-gas emissions from soils increased by earthworms.Nature Climate Change3(3):187‑194. https://doi.org/10.1038/nclimate1692
- Recommendations for establishing global collaborative networks in soil ecology.SOIL ORGANISMS91(3 SE - ARTICLES). https://doi.org/10.25674/so91iss3pp73
- Measuring biological diversity.John Wiley & Sons
- Global effects of land use on local terrestrial biodiversity.Nature520(7545):45‑50. https://doi.org/10.1038/nature14324
- Has land use pushed terrestrial biodiversity beyond the planetary boundary? A global assessment.Science353(6296):288‑291. https://doi.org/10.1126/science.aaf2201
- Global Soil Biodiversity Atlas.European Commission[ISBN978-92-79-48168-0] https://doi.org/10.2788/2613
- Global hotspots of species richness are not congruent with endemism or threat.Nature436(7053):1016‑1019. https://doi.org/10.1038/nature03850
- Scenarios for Global Biodiversity in the 21st Century.Science330(6010):1496‑1501. https://doi.org/10.1126/science.1196624
- Land-use effects on local biodiversity in tropical forests vary between continents.Biodiversity and Conservation26(9):2251‑2270. https://doi.org/10.1007/s10531-017-1356-2
- Red list of a black box.Nature Ecology & Evolution1(4). https://doi.org/10.1038/s41559-017-0103
- The effect of fragment area on site-level biodiversity.Ecography41(7):1220‑1231. https://doi.org/10.1111/ecog.02956
- Global distribution of earthworm diversity.Science366(6464):480‑485. https://doi.org/10.1101/587394
- The biodiversity of species and their rates of extinction, distribution, and protection.Science344(6187). https://doi.org/10.1126/science.1246752
- Detecting macroecological patterns in bacterial communities across independent studies of global soils.Nature Microbiology3(2):189‑196. https://doi.org/10.1038/s41564-017-0062-x
- Toward a global platform for linking soil biodiversity data.Frontiers in Ecology and Evolution3https://doi.org/10.3389/fevo.2015.00091
- R: A language and environment for statistical computing.R Foundation for Statistical Computing,Vienna, Austria.
- The Shared Socioeconomic Pathways and their energy, land use, and greenhouse gas emissions implications: An overview.Global Environmental Change42:153‑168. https://doi.org/10.1016/j.gloenvcha.2016.05.009
- Soil net nitrogen mineralisation across global grasslands.Nature Communicationshttps://doi.org/10.1038/s41467-019-12948-2
- The global distribution of tetrapods reveals a need for targeted reptile conservation.Nature Ecology & Evolution1(11):1677‑1682. https://doi.org/10.1038/s41559-017-0332-2
- Latitudinal gradients: Higher predation risk for insect prey at low latitudes and elevations.Science356(6339):742‑744. https://doi.org/10.1126/science.aaj1631
- Biodiversity of Collembola and their functional role in the ecosystem.Biodiversity and Conservation7(9):1207‑1219. https://doi.org/10.1023/A:1008887817883
- Mapping earthworm communities in Europe.Applied Soil Ecology97:98‑111. https://doi.org/10.1016/j.apsoil.2015.08.015
- Global biodiversity scenarios for the year 2100.Science287(5459):1770‑1774. https://doi.org/10.1126/science.287.5459.1770
- Cause and Correlation in Biology A User's Guide to Path Analysis, Structural Equations and Causal Inference.20.ambridge University Press[ISBN0511017723] https://doi.org/10.1017/CBO9780511605949
- Building a global database of soil microbial biomass and function: a call for collaboration.SOIL ORGANISMS91(3 SE - ARTICLES). https://doi.org/10.25674/so91iss3pp140
- Biodiversity at multiple trophic levels is needed for ecosystem multifunctionality.Nature536(7617):456‑459. https://doi.org/10.1038/nature19092
- Global diversity and geography of soil fungi.Science346(6213):1256688‑1256688. https://doi.org/10.1126/science.1256688
- A mid-term analysis of progress toward international biodiversity targets.Science346(6206):241‑244. https://doi.org/10.1126/science.1257484
- Soil nematode abundance and functional group composition at a global scale.Nature572(7768):194‑198. https://doi.org/10.1038/s41586-019-1418-6
- Earthworms increase plant production: a meta-analysis.Scientific Reports4(2). https://doi.org/10.1038/srep06365
- Extinction debt of forest plants persists for more than a century following habitat fragmentation.Ecology87(3):542‑548. https://doi.org/10.1890/05-1182
- Global meta-analysis reveals no net change in local-scale plant biodiversity over time.Proceedings of the National Academy of Sciences110(48):19456‑19459. https://doi.org/10.1073/pnas.1312779110
- Estimates of local biodiversity change over time stand up to scrutiny.Ecology98(2):583‑590. https://doi.org/10.1002/ecy.1660
- Extinction risk of soil biota.Nature Communications6https://doi.org/10.1038/ncomms9862
- Contributions of a global network of tree diversity experiments to sustainable forest plantations.Ambio45(1):29‑41. https://doi.org/10.1007/s13280-015-0685-1
- Soil biodiversity and soil community composition determine ecosystem multifunctionality.Proceedings of the National Academy of Sciences111(14):5266‑5270. https://doi.org/10.1073/pnas.1320054111
- Global decomposition experiment shows soil animal impacts on decomposition are climate-dependent.Global Change Biology14(11):2661‑2677. https://doi.org/10.1111/j.1365-2486.2008.01672.x
- Perspective Soil biodiversity and human health.Nature528:69‑76. https://doi.org/10.1038/nature15744
- A global map of travel time to cities to assess inequalities in accessibility in 2015.Nature553(7688):333‑336. https://doi.org/10.1038/nature25181
- Abundance of common species, not species richness, drives delivery of a real-world ecosystem service.Ecology Letters18(7):626‑635. https://doi.org/10.1111/ele.12424
- Earthworms facilitate carbon sequestration through unequal amplification of carbon stabilization compared with mineralization.Nature Communications4(1). https://doi.org/10.1038/ncomms3576
Supplementary material
Sampling protocol to ensure standardised methods for researchers sampling soil macrofauna (and ecosystem functions) in a new site.