|
Research Ideas and Outcomes :
Research Article
|
|
Corresponding author: Nursigit Bintoro (nursigit@ugm.ac.id)
Received: 24 Jul 2020 | Published: 31 Jul 2020
© 2020 Sugeng Sugiharto, Nursigit Bintoro, Joko Karyadi, Yudi Pranoto
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation: Sugiharto SP, Bintoro N, Karyadi JNW, Pranoto Y (2020) Supercritical carbon dioxide pasteurization to reduce the activity of muscle protease and its impact on physicochemical properties of Nile tilapia. Research Ideas and Outcomes 6: e56887. https://doi.org/10.3897/rio.6.e56887
|
|
Abstract
The studies of the effect of supercritical carbon dioxide (scCO2) pasteurization on solid food from fish origin are scarcely available. This study was intended to address that gap by investigating the effect of scCO2 on the reduction of muscle protease activity and its impact on physicochemical properties of the Nile tilapia. Tilapia were exposed to CO2 pressure at 70, 75, 80, 85, and 90 bar; temperature at 40 °C; and holding time for 15 min. This study discovered that 80 bar was the minimum pressure to achieve half residual activity of muscle protease and two logs reductions of microbial counts. The applications of 80 and 85 bar were found to achieve significant reduction of tilapia muscle protease activity while still maintained acceptable textural properties. Both 80 and 85 bar were found to be effective to inhibit softening development of tilapia fillet during 14 days of chilled storage. Eighty-five bar and 15 min CO2 pasteurization was considered as maximum level of CO2 pressure that tilapia could withstand without degrading its texture significantly.
Keywords
tilapia, supercritical, CO2, protease, physicochemical
Introduction
High-pressure carbon dioxide process (HPCD) has gained considerable interests in the scientific fields. Since the 1980s, HPCD has been increasingly reported as a promising technique to induce the pasteurizing or sterilizing effects to solid and liquid foodstuffs. Compared to liquid foods, the HPCD process that is applied to solid foods has been under-researched due to the complexity of the biological matrices, which could make CO2 bactericidal action more arduous, and the lack of information about the inactivation mechanism which is almost obscure and scarcely studied (
Most studies of scCO2 effects on solid food from fish sources were mostly focused on the bactericidal efforts.
Though reports of inactivation of isolated enzymes are available, studies of the effect of HPCD on muscle enzymes are rarely found. The effects of the high-pressure processing on muscle proteases were mostly reported from the High Pressure Processing (HPP) application.
In this study, Nile tilapia (Oreochromis niloticus) was used as a solid food because tilapia is the second most produced freshwater fish after carps. Global production of tilapia was 5.7 million tonnes, which was 7.4% of the total global aquaculture production in 2015. Ten percent of global tilapia products were traded in international market (
The objective of this study, therefore, was to evaluate the HPCD process on muscle protease and its effect on selected physicochemical properties of tilapia fillet, particularly on the color and firmness of fish. Protease, a causing factor for meat softening during storage, even under low temperature, was examined. In addition, color and firmness, which are the determining factors for consumers’ acceptance in purchasing raw fishes, were investigated. This work was intended to search the balance between good inactivation rate of muscle protease and keeping changes of fish physicochemical properties as low as possible.
The fish itself was not intended for direct human consumption. This HPCD pasteurization was intended to reduce quality degradation during chilled storage, and eventually, extend shelf life. For human consumption, this HPCD treated fish should be handed over to further processing, such as heat cooking, fermentation, etc.; which would depend on intended final products.
Material and methods
Material
The Nile tilapia fishes (weight ranging from 100 to 150 g each) were purchased alive from the local market. All fishes were slaughtered, gutted, headed, and cleaned with tap water. These cleaned fishes were cut into two pieces, inserted into a polyethylene plastic pouch (20 × 15 cm), and subsequently placed into the pressure vessel. The plastic pouch tips were folded and unsealed to allow CO2 exchange as well as prevented any external solid or liquid entering the pouch container.
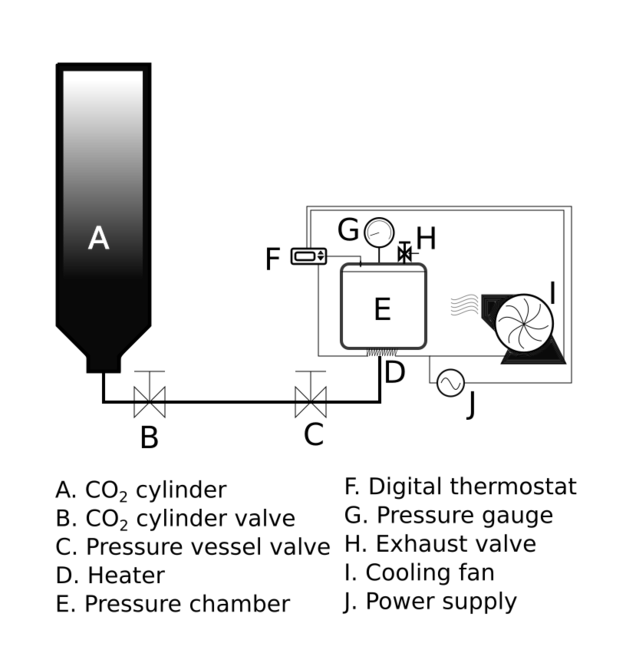
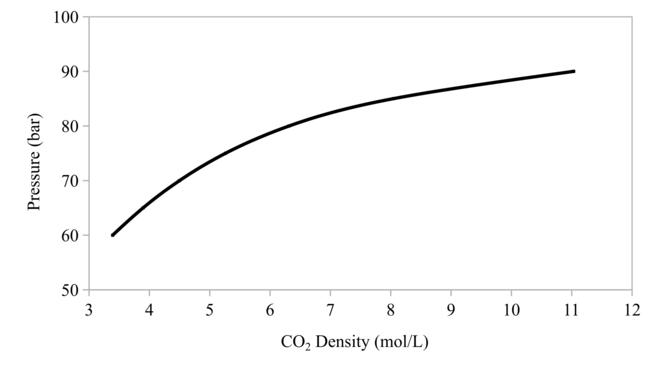
The pressure vessel was developed in Gadjah Mada University with gross volume of 500 mL. Its lid was equipped with 180 bar analog pressure gauge with five bar resolution. A calibrated digital thermostat (Elitech STC-3000) with a 10 KΩ negative-temperature-coefficient (NTC) thermistor was used to control barrel temperature of the pressure vessel. Food grade CO2 was supplied into the pressure chamber by inverting the 25 kg-type CO2 cylinder. This equipment could provide a maximum pressure of 90 bar at 40 °C (CO2 density = 0.486 g/mL at 40 °C), which was the upper limit of this experiment. The schematic installation of the pressure vessel is shown in Fig.
Methods
CO2 pressurization
Liquid CO2 was supplied into the pressure vessel by inverting the CO2 cylinder upside and down. During the filling process, exhaust orifice was opened slightly to remove non-CO2 gas and reduce pressure chamber temperature simultaneously. Experimental pressure was achieved by raising barrel temperature. In order to prevent overpressure, the pressure was maintained by releasing excess CO2 through exhaust orifice when temperature and pressure approached experimental setting. The time required to raise the temperature from 30 °C to 40 °C was ±10 min. Decompression time was set for 2–3 min. Rapid decompression was found destroyed the texture of fish during previous preliminary study.
Crude enzyme extract
The enzyme extraction process followed the method proposed by
Residual protease activity measurement
Protease assay followed the method of
Aerobic microbial counts determination
Ten grams of samples in 90 mL of 0.85% NaCl were homogenized in bag stomacher (Interscience BagMixer 400P) for 5 min. Serial dilution of untreated samples was performed to find appropriate dilution level. One mL of diluted sample was mixed with melted plate count agar (44–46 °C) and incubated at 35 °C for 48 hrs. After incubation, the petri dishes of the incubated sample were placed on top of backlight source and digitally photographed with resolution of 10 million pixels. All the images were processed, cropped, and scaled at 2772 pixels horizontally. OpenCFU software was used to enumerate the colony forming unit (CFU) (
Texture measurements
TA.XT plus texture analyzer (Stable Microsystem Inc.) with 50 kg load cell was used to measure textural property of fish chunks. Fish chunks with 40 mm width were compressed to 50% of their original height using a 36 mm diameter cylindrical probe. The compression speed was set at 2 mm/s. The readings were recorded with an interval of 0.005 s.
Color readings
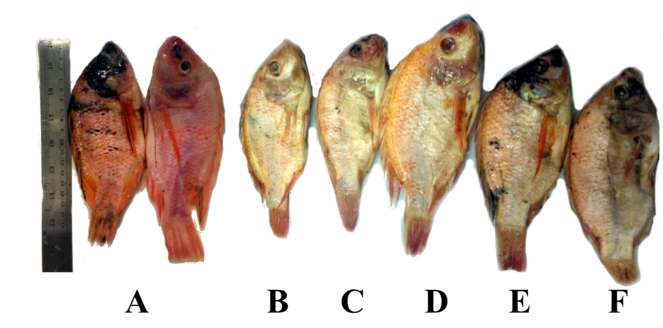
Fish muscle was filleted, and the inner part of fillet surface color was read using a handheld colorimeter (Color muse, Variable Inc). Subcutaneous or skin color could not be used for color readings due to uneven skin pigmentation. The skin appearance of pre and post-treated whole fish is displayed in Fig.
Before color reading, colorimeter had to be calibrated against supplied standard white. Each fillet was read on three different spots. Color was expressed in CIE L* (whiteness or brightness), a* (redness/greenness), and b* (yellowness/blueness) coordinates. Total Color differences (ΔΕ00) were calculated according to CIEDE-2000 standard (
Proximate analysis and pH
Fat content was measured through soxhlet methods (
Ash content was calculated by furnace burning at 600 °C for 6 hours. Moisture content was analyzed by overnight (12 hours) oven drying at 105 °C. Lastly, pH was determined by immersion of pH meter electrode (Metler Toledo FiveEasy F20) into the suspension of 1:9 sample in deionized water.
Observation of muscle softening during chilled storage
During storage under frozen and chilled temperature, protease is responsible for muscle softening. Beside muscle protease, spoilage microorganism also contributes protease into the fish tissue that could worsen muscle softening.
The fillets, which their texture were not significantly different compared to control, were monitored its softening development under chilled storage. To minimize the effect of decomposition due to spoilage microorganisms, fish chunks were stored in chilled temperature (4 °C) in 14 days storage. Seven points observations were taken, on initial day (day 1), 3rd, 6th, 8th, 10th, 12th and 14th day. Mean values were taken from means of three replications. Each replication was conducted on individual fish. Observations were chosen from treatments that retained texture, which were not significantly different than that of initial untreated samples. To measure the progression of muscle softening, fillet hardness was measured on each observation day.
The percentage of softening was stated as a ratio of observed hardness of the pasteurized samples (H) to initial hardness of the untreated samples (H0).
Experimental design and statistical analysis
Due to the equipment’s limitation, which was limited at the maximum of 90 bar at 40 °C (the maximum density was 0.4855 g/mL CO2 at 40 °C), five points pressures from 70 to 90 bar at 40 °C with 5 bar intervals were applied. Subcritical phase was 70 bar, and supercritical phases were 75, 80, 85, and 90 bar, respectively. The observation parameters were protease residual activity (%), color (L*a*b color space), hardness, weight, and proximate analysis. The performed proximate analysis was fat content, protein content, and ash content. Each replication was performed from different fish.
Results were expressed as mean values ± standard deviation of three replications. One way analysis of variance (ANOVA) was carried out to examine the significance of treatments (p < 0.05). After ANOVA, New Duncan Multiple Range Test was used to perform pairwise comparisons. All statistical works were performed with R statistical language (
Results
Effect of CO2 pressure and density on muscle protease activity and microbial counts reduction
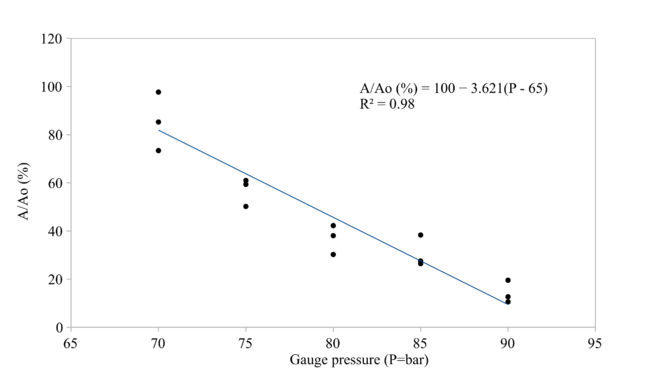
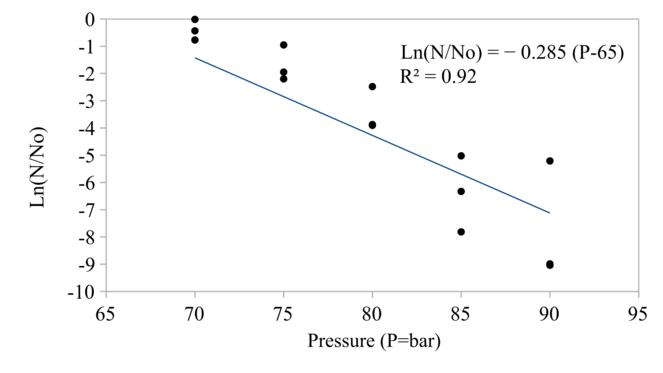
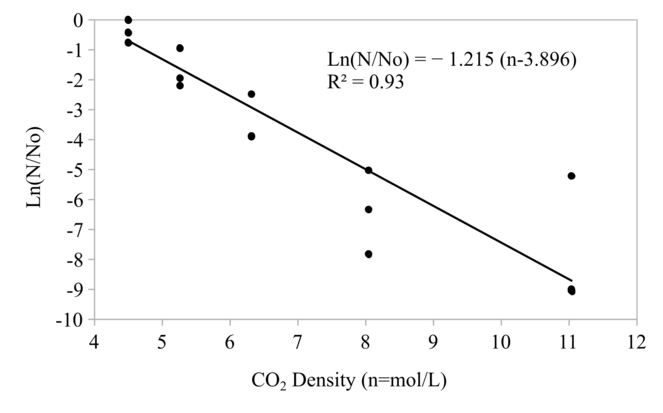
The CO2 pressure and density affected the reduction of protease activity and microbial loads significantly as shown in Fig.
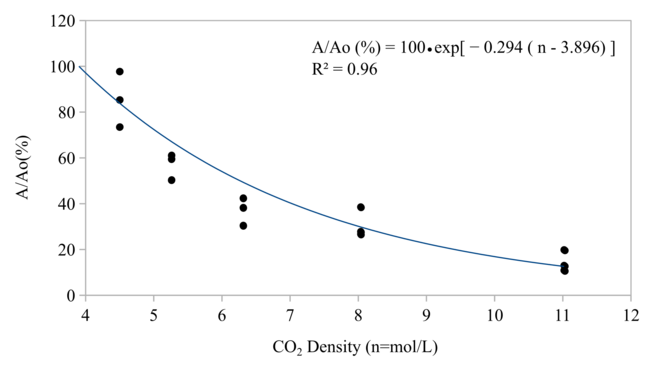
Since the relationship between pressure and density of CO2 at 40 °C is not linear as shown in Fig.
As shown in Fig.
Effect of CO2 pressure on muscle texture
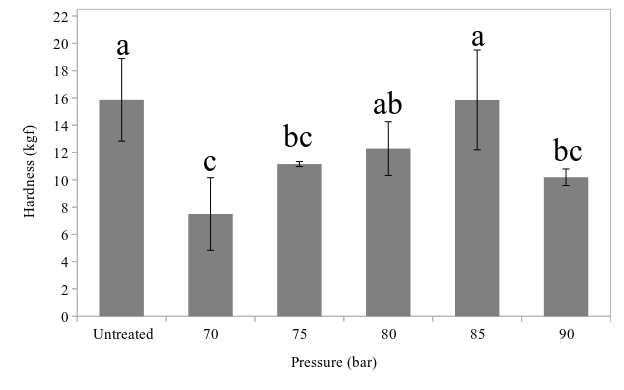
The relationship between pressure and treated fillet texture was not linear, as shown in Fig.
In the range from 70 to 90 bar, only 80 and 85 bar treatments could retain texture that were not significantly different than that of untreated samples. All other treatments showed significant softening compared to untreated samples. Hence, 85 bar and 15 min HPCD treatment was considered as maximum level of CO2 pressure that tilapia fillet could withstand without suffering severe textural degradation.
Effect of CO2 on fillet color
The HPCD pressure was found to have very significant effect on Lightness values (L*), as shown in Table
|
Pressure (Bar) |
L* |
a* |
b* |
Color difference† (ΔE00) |
|
untreated fillet |
53.01 ± 1.89c |
8.50 ± 1.87a |
3.32 ± 2.74a |
- |
|
70 |
78.48 ± 3.18a |
7.48 ± 1.00a |
9.37 ± 1.70a |
21.43 ± 2.000 |
|
75 |
77.79 ± 0.76a |
8.87 ± 2.72a |
7.19 ± 2.38a |
20.65 ± 0.277 |
|
80 |
75.05 ± 5.46ab |
13.13 ± 2.10a |
8.99 ± 1.87a |
19.12 ± 3.548 |
|
85 |
69.77 ± 6.36b |
9.95 ± 2.78a |
10.87 ± 6.54a |
15.77 ± 5.59 |
|
90 |
78.95 ± 4.65a |
9.02 ± 4.65a |
9.36 ± 5.83a |
22.11 ± 2.25 |
a-d means with the same superscript letter were not significantly different
† against untreated samples.
For consumers’ perception, color is one of the most important sensory characteristics of fish muscles in determining their purchasing decision (
Effect of CO2 on proximate analysis and pH
As shown in Table
Effect of CO2 pressure at 40 °C on proximate values of fillet and its post-pressurization pH.
|
Pressure (bar) |
Weight loss |
pH |
Moisture |
Fat contents |
Protein contents |
Ash contents |
|
Untreated |
— |
8.49 ± 0.08a |
74.60 ± 3.73a |
2.03 ± 0.86b |
21.19 ± 0.95b |
0.51 ± 0.07ab |
|
70 |
19.22 ± 3.63a |
8.06 ± 0.04b |
72.32 ± 2.00a |
5.13 ± 1.17a |
21.64 ± 0.08ab |
0.20 ± 0.28b |
|
75 |
17.75 ± 2.58a |
7.90 ± 0.14bc |
73.32 ± 0.91a |
4.42 ± 1.35a |
21.92 ± 0.03ab |
0.87 ± 0.24a |
|
80 |
15.92 ± 2.50a |
7.81 ± 0.09c |
75.42 ± 2.59a |
1.99 ± 0.47b |
19.95 ± 2.80b |
0.85 ± 0.48a |
|
85 |
18.75 ± 2.55a |
8.54 ± 0.20a |
72.93 ± 2.70a |
4.33 ± 1.50b |
22.29 ± 1.15 ab |
1.19 ± 0.09a |
|
90 |
18.29 ± 2.44a |
8.09 ± 0.12b |
72.25 ± 1.67a |
2.12 ± 0.79b |
24.01 ± 0.80a |
0.62 ± 0.23ab |
a-c Means with the same superscript letter were not significantly different.
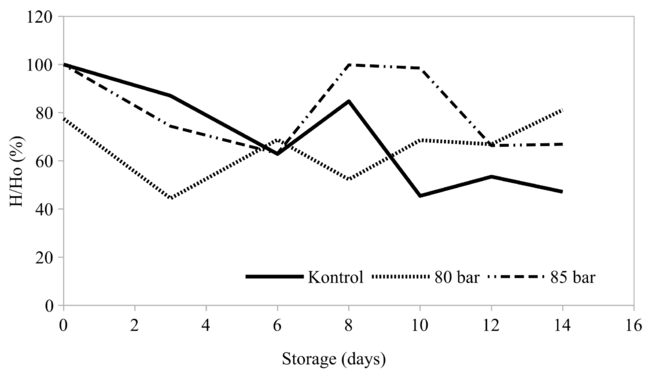
Compared to the untreated samples, 80 and 85 bar treatments did not experience hardness change significantly (Fig.
Effect of HPCD on softening development during 14 days of storage
As shown in Fig.
On the final day of observation, untreated samples lost 52.9% of its initial hardness. This finding was in agreement with the softening of climbing perch (Anabas testudineus), which lost 50.1% of its initial hardness after 15 days stored in ice (
Discussion
Pasteurization effect of CO2 pressure and density on protease inactivation and microbial counts reduction
In this work, the CO2 pressure significantly affected the reduction of protease activity and microbial counts. The mechanisms of protease inactivation by HPCD were proposed by several researchers. The pressure itself is not adequate to reduce protease activity as reported by HPP application.
The solubility of CO2 and its dissolution rate into the cell cytoplasm and muscle tissue liquid when HPCD applied on muscle tissue is still unknown and need further investigation. The cell size of eukaryotes is within 10-100 μm (
It could be expected that if subjected to HPCD pressure higher than 90 bar (at 40 °C), the fish texture would be deteriorated severely. Since this work was to search the balance between low residual protease activity and acceptable textural properties, complete inactivation of protease should be avoided. Elimination of muscle protease activity would result in severe degradation of fillet textural properties. In this regard, 80 and 85 bar of HPCD pressure at 40 °C can be considered as optimum pressure to reduce activity of muscle protease, as well as maintaining good textural properties of tilapia fillet.
Beside enzymatic activity, microbial activity also plays significant role on food deterioration. The bactericidal effect of scCO2 could be contributed by one or more mechanisms. It could be cell disruption (
Acceptable residual microbial counts in this work was achieved by 90 bar application. Unfortunately, 90 bar application was failed to maintain acceptable texture (Fig.
Maximum aerobic microbial counts of frozen raw fish was recommended at 5·105 CFU/g (
Effect of CO2 pressure on selected fish physicochemical properties
The effect of HPCD pressure on texture was found significant but did not produce stable trend. Some researchers reported the effect of pressure of scCO2 on texture of some seafoods qualitatively (
At 90 bar application, scCO2 pressure resulted severe adverse effect on textural property and muscle integrity. Maximum pressure for the fish fillet could withstand CO2 pressure was considered at 85 bar. Ninety bar CO2 pressure or more might be justified for cooking application to eliminate completely both enzymatic and microbiological activities. HPCD might be a prospective application for the meat better than for the fish. Consumers preference for raw meat is freshness and tenderness (
Compared to HPP, color change induced by HPCD was initiated at much lower pressure. Color change of HPP treatment on carp fillet was reported to be initiated at 1,000 bar (
The color changes affected by high pressure is likely due to degradation of muscle myoglobin. The required temperature to denature myoglobin of tilapia significantly was reported at 60 °C (
As it was explained previously, scCO2 extracted cellular components and in this work, also squeezed out these components out of the cells and muscle tissues. This extraction and squeezing effect reduced fillet mass, especially during decompression stage. Lipids also well known dissolved in scCO2.
Brief comparison of HPCD and HPP, the advantage and disadvantage
In the non-thermal food processing technology, HPP and HPCD are two novel technologies that use high pressure. HPP employs ultrahigh hydrostatic pressure, up to 10,000 bar (
The operational pressure of HPCD, which is significantly lower than that of HPP, requires less pressure resistant assembly than that of HPP equipments. HPP pasteurization requires 7,000 bar or more to achieve permanent inactivation of enzymes and need ultra-high pressure capable installation. Though HPCD requires lesser pressure resistant installation than that of HPP installation, it requires seal that impenetrable from scCO2 diffusion. Soft metal seal such as aluminum might be impenetrable from scCO2 but requires much more tightening force to be well sealed; while polymer seals, e.g. rubber and silicone, could not withstand scCO2 penetration during long or repetitive HPCD application. HPP requires much more rugged installation than that of HPCD, including its seals, while HPCD requires lesser pressure resistant installation but need impenetrable seal from high diffusivity CO2. If the same product is pasteurized by both HPP and HPCD, HPP requires more pumping power to deliver hydraulic pressure but need lower material input than that of HPCD, while the HPCD requires less power input to pump CO2 but need more material input than that of HPP. Furthermore, products subjected to HPP do not need post treatment after pressurization, since it is already packed in final packaging; while HPCD products need immediate post pressurization handling since its packaging is remaining open after pasteurization.
Conclusion
This study discovered that HPCD technique was able to demonstrate its potential to reduce muscle protease activity and microbial contamination of raw tilapia fillet, while simultaneously maintained some of its physicochemical qualities. Eighty-five bar at 40 °C for 15 min was considered as the optimum pressure to reduce muscle protease activity without reducing its textural quality significantly. The softening development of 80 and 85 bar treated fillets were found slower than that of untreated fillets during 14 days chilled storage. Prior to HPCD pasteurization, microbial counts on raw fishes should be reduced in order to comply with food safety regulation.
The residual activity of both protease and microbial counts was found as the function of CO2 density and follow first order reaction. The CO2 density is the main reason for much lower necessary pressure of HPCD application when compared to HPP pressure.
Funding program
This works is funded partially by Ministry of Research, Technology, and Higher Education; and Thesis Recognition Program, Gadjah Mada University, Grant Number 3395/UN1/DITLIT/DIT-LIT/LT/2019.
References
-
Protease (Aspergillus alkaline proteinase, EC 3.4.21.63). http://cy-bio.com/Administrator/Order/20091222125934b.pdf. Accessed on: 2016-9-16.
-
Application of high hydrostatic pressure to control enzyme related fresh seafood texture deterioration.Food Research International29:569‑575. https://doi.org/10.1016/s0963-9969(96)00012-9
-
SNI 7388-2009 tentang Batas Maksimum Cemaran Mikroba dalam Pangan.[SNI 7388-2009: Maximum limit of microbial contamination on food].Badan Standarisasi Nasional,Jakarta,41pp. [InIndonesian].
-
Protease stability in bovine milk under combined thermal-high hydrostatic pressure treatment.Innovative Food Science & Emerging Technologies10(3):314‑320. https://doi.org/10.1016/j.ifset.2009.01.003
-
Campbell - Biology.7th Edition.Pearson Education,1312pp. [ISBN080537146X] https://doi.org/10.1083/jcb.200503162
-
Determination of fat content. In:Food analysis laboratory manual.2nd Edition.Springer,New York,29-37pp. [ISBN9780123693976]. https://doi.org/10.1007/978-1-4419-1463-7
-
Effect of carbon dioxide on the inactivation of florida spiny lobster polyphenol oxidase.Journal of the Science of Food and Agriculture61(2):253‑259. https://doi.org/10.1002/jsfa.2740610219
-
Thermal stability and denaturation rate of myoglobin from various species of fish.Fisheries Science70(2):293‑298. https://doi.org/10.1111/j.1444-2906.2003.00803.x
-
Review of dense phase CO2 technology: Microbial and enzyme Inactivation, and effects on food quality.Journal of Food Science71(1). https://doi.org/10.1111/j.1365-2621.2006.tb12397.x
-
Effect of supercritical carbon dioxide processing on Vibrio parahaemolyticus in nutrient broth and in oysters (Crassostrea gigas).Journal of Food Science and Technology55(10):4090‑4098. https://doi.org/10.1007/s13197-018-3335-3
-
High pressure carbon dioxide pasteurization of solid foods: Current knowledge and future outlooks.Trends in Food Science & Technology22(8):427‑441. https://doi.org/10.1016/j.tifs.2011.04.009
-
Bursting bacteria by release of gas pressure.Nature167(4236):33‑34. https://doi.org/10.1038/167033b0
-
OpenCFU, a new free and open-source software to count cell colonies and other circular objects.PLOS One8(2). https://doi.org/10.1371/journal.pone.0054072
-
Solubility in supercritical carbon dioxide.CRC-Press,Boca Raton-FL,357-368pp.
-
Solubilities of pure lipids in supercritical carbon dioxide.The Journal of Supercritical Fluids5(2):101‑106. https://doi.org/10.1016/0896-8446(92)90026-g
-
Food preservation by high pressure.Journal für Verbraucherschutz und Lebensmittelsicherheit5(1):73‑81. https://doi.org/10.1007/s00003-009-0311-x
-
High pressure processing of foods: An overview.Emerging Technologies for Food Processing3‑32. https://doi.org/10.1016/b978-012676757-5/50003-7
-
Tilapia players debate industry future. https://www.seafoodsource.com/features/tilapia-players-debate-industry-future. Accessed on: 2019-9-21.
-
Membrane damage and enzyme inactivation of Lactobacillus plantarum by high pressure CO2 treatment.International Journal of Food Microbiology63:19‑28. https://doi.org/10.1016/s0168-1605(00)00393-7
-
Traditional microbiological quality control. http://www.fao.org/3/T1768E/T1768E04.htm. Accessed on: 2019-5-13.
-
Microorganisms in foods 2. Sampling for microbiological analysis: Principles and specific applications.2nd Edition.University of Toronto Press,Toronto,293pp. URL: https://seafood.oregonstate.edu/sites/agscid7/files/snic/sampling-for-microbiological-analysis-principles-and-specific-applications-icmsf.pdf [ISBN0802056938]
-
Inactivation of Enzymes and Decomposition of α-Helix Structure by Supercritical Carbon Dioxide Microbubble Method.Journal of Agricultural and Food Chemistry44(9):2646‑2649. https://doi.org/10.1021/jf9602075
-
Inactivation of enzymes in an aqueous solution by micro-bubbles of supercritical carbon dioxide.Bioscience, Biotechnology, and Biochemistry59(4):628‑631. https://doi.org/10.1271/bbb.59.628
-
Optimization of microbial inactivation of shrimp by dense phase carbon dioxide.International Journal of Food Microbiology156(1):44‑49. https://doi.org/10.1016/j.ijfoodmicro.2012.02.020
-
Sterilization of microorganisms with supercritical carbon dioxide.Agricultural and Biological Chemistry51(2):407‑412. https://doi.org/10.1080/00021369.1987.10868053
-
Effect of hydrostatic pressure and holding time on physicochemical quality and microbial inactivation kinetics of black tiger shrimp (Penaeus monodon).Innovative Food Science & Emerging Technologies33:47‑55. https://doi.org/10.1016/j.ifset.2015.12.002
-
Effects of high-pressure processing on proteolytic enzymes and proteins in cold-smoked salmon during refrigerated storage.Food Chemistry90(4):541‑548. https://doi.org/10.1016/j.foodchem.2004.05.015
-
Influence of high pressure homogenization on commercial protease from Rhizomucor miehei: Effects on proteolytic and milk-clotting activities.LWT - Food Science and Technology63(1):739‑744. https://doi.org/10.1016/j.lwt.2015.03.020
-
Thermophysical properties of fluid systems. In:NIST Chemistry WebBook, NIST Standard Reference Database Number 69. https://doi.org/10.18434/T4D303
-
Disintegration of yeast cells by pressurized carbon dioxide.Biotechnology Progress7(3):201‑204. https://doi.org/10.1021/bp00009a001
-
An improved method for disruption of microbial cells with pressurized carbon dioxide.Biotechnology Progress8(2):165‑166. https://doi.org/10.1021/bp00014a012
-
NIR spectroscopy and imaging techniques for evaluation of fish quality— a review.Applied Spectroscopy Reviews48(8):609‑628. https://doi.org/10.1080/05704928.2013.775579
-
High pressure processing of fresh meat — Is it worth it?Meat Science95(4):897‑903. https://doi.org/10.1016/j.meatsci.2013.03.025
-
Effects of high pressure on colour and texture of fish.High Pressure Research19:109‑115. https://doi.org/10.1080/08957950008202543
-
Bacteriological Analytical Manual. Chapter 3: Aerobic Plate Count. https://www.fda.gov/food/laboratory-methods-food/bam-aerobic-plate-count. Accessed on: 2017-11-16.
-
Supercritical fluid extraction.2nd Edition,53 Volume.Butterworth-Heinemann,Newton, MA,1-16pp. [ISBN0080518176] https://doi.org/10.1016/B978-0-08-051817-6.50004-7.
-
Control of eschericia coli O157:H7, generic Escherichia coli, and Salmonella spp. on beef trimmings prior to grinding using a controlled phase carbon dioxide (CPCO2) system.Kansas State University,Kansas,142pp. URL: https://krex.k-state.edu/dspace/bitstream/handle/2097/231/CarlosTanus2006.pdf?sequence=1&isAllowed=y
-
Reducing oyster-associated bacteria levels using supercritical fluid CO2 as an agent of warm pasteurization.International Journal of Food Microbiology138:63‑70. https://doi.org/10.1016/j.ijfoodmicro.2009.11.012
-
Prediction of pH in model systems pressurized with carbon dioxide.Biotechnology Progress8(2):149‑154. https://doi.org/10.1021/bp00014a009
-
Aquaculture production and trade trends: carp, tilapia and shrimp, FAO RAP. http://www.fao.org/fi/static-media/MeetingDocuments/WorkshopAMR17/presentations/28.pdf. Accessed on: 2017-11-15.
-
Protein nitrogen determination. In:Food analysis laboratory manual.2nd Edition.Springer,New York,39-45pp. [ISBN978-1-4419-1462-0]. https://doi.org/10.1007/978-1-4419-1463-7_5
-
Myoglobin secondary structure effects of pH - circular dichroism In: JASCO Applications Book. https://jascoinc.com/applications/circular-dichroism-myoglobin-ph-titration/. Accessed on: 2020-6-08.
-
R: A language and environment for statistical computing. https://www.R-project.org/. Accessed on: 2016-2-10.
-
Physicochemical changes induced in carp (Cyprinus carpio) fillets by high pressure processing at low temperature.Innovative Food Science & Emerging Technologies7:13‑18. https://doi.org/10.1016/j.ifset.2005.06.006
-
The CIEDE2000 color-difference formula: Implementation notes, supplementary test data, and mathematical observations.Color Research & Application30(1):21‑30. https://doi.org/10.1002/col.20070
-
A new equation of state for carbon dioxide covering the fluid region from the triple-point temperature to 1100 K at pressures up to 800 MPa.Journal of Physical and Chemical Reference Data25(6):1509‑1596. https://doi.org/10.1063/1.555991
-
Proteases in fish and shellfish: Role on muscle softening and prevention.International Food Research Journal21(2):433‑445. URL: http://www.ifrj.upm.edu.my/21%20(02)%202014/2%20IFRJ%2021%20(02)%202014%20Sriket%20014.pdf
-
Feasibility study on CO2 micro-bubble storage (CMS).Energy Procedia37:6002‑6009. https://doi.org/10.1016/j.egypro.2013.06.528
-
Pasteurization of food by hydrostatic high pressure: chemical aspects.Zeitschrift für Lebensmittel-Untersuchung und -Forschung200(1):3‑13. https://doi.org/10.1007/bf01192901
-
Advances in high-pressure processing of fish muscles.Food Engineering Reviews7(2):109‑129. https://doi.org/10.1007/s12393-014-9084-9
-
Assessment of the textural variation of iced stored Anabas testudineus (Bloch, 1792) muscle tissue with emphasis on their collagen and myofibrillar protein content.Journal of Food Science and Technology54(8):2512‑2518. https://doi.org/10.1007/s13197-017-2695-4
-
Bacterial effect of high pressure CO2 treatment on foods spiked with Listeria or Salmonella.Journal of Food Protection54(3):189‑193. https://doi.org/10.4315/0362-028x-54.3.189
-
Application of high pressurization to fish meat: Changes in the physical properties of carp skeletal muscle resulting from high pressure thawing.High Pressure Bioscience and Biotechnology, Proceedings of the International Conference on High Pressure Bioscience and Biotechnology369‑374. https://doi.org/10.1016/s0921-0423(06)80062-6
-
The size of sonoporation pores on the sell membrane.Ultrasound in Medicine & Biology35(10):1756‑1760. https://doi.org/10.1016/j.ultrasmedbio.2009.05.012
Original paper was published in 1995 and republished as electronic paper in 2014