|
Research Ideas and Outcomes :
Grant Proposal
|
|
Corresponding author: Hanna Ćwiek-Kupczyńska (hcwi@igr.poznan.pl), Paweł Krajewski (pkra@igr.poznan.pl)
Received: 02 Sep 2021 | Published: 03 Sep 2021
© 2021 Hanna Ćwiek-Kupczyńska, Paweł Krajewski
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Ćwiek-Kupczyńska H, Krajewski P (2021) Polish network of research infrastructure for plant phenotyping. Research Ideas and Outcomes 7: e73858. https://doi.org/10.3897/rio.7.e73858
|
|
Abstract
This document is an edited version of the original application for inclusion of a strategic research infrastructure project in the Polish Roadmap for Research Infrastructures. The application entitled "Polish network of research infrastructure for plant phenotyping" was submitted to the Polish Ministry of Science and Higher Education in June 2018; the project was not included in the Roadmap published in January 2020. The original document did not contain this abstract.
Keywords
research infrastructures, application, plant phenotyping
Applicant details
Name, registered office and address:
Institute of Plant Genetics, Polish Academy of Sciences, Strzeszyńska 34, 60-479 Poznań, Poland
Full name of the head of the unit:
Prof. dr hab. Bogdan Wolko, Director
1. Description of the underlying goals and objectives of the project, taking into account the project’s contribution to expand and increase capability of the national scientific community as well as to improve competitiveness and increase the level of technological innovation of the Polish economy.
The aim of the project is building and running of a distributed research infrastructure named "Polish network of research infrastructure for plant phenotyping", called hereafter the Network. The Network will comprise the existing research infrastructure dedicated by the partners and the infrastructure that will be built after creation of the Network. The motivation for creating the Network is the urgent need to support the maintenance and development of the national research potential in the area of plant phenotyping, which is crucial for the progress and success of plant research. The Network will stimulate and coordinate the national collaboration on introduction of innovative phenotyping methods and represent Polish plant research community in the European infrastructural platforms. The partners of the Network will be scientific units (universities, institutes of Polish Academy of Sciences, state research institutes) and seed and breeding companies, which will join the agreement on jointly elaborated conditions.
The phenotype is defined as the complex of observed features of plants shaped by the interactive action of the genotype and the environment. The main group of phenotypic traits is made of the features that can be observed by the application of classical measurement methods on whole plants, i.e., the traits describing morphology, architecture, phenology and productivity; contemporary measurement methods allow to extend this definition to the description of tissues, organs or to descriprion of canopies and natural populations. Modern methods also allow to measure phenotypic traits that are physiological or biochemical parameters. The phenotype is observed with the aim of learning the ultimate effects of events taking place at the molecular level and the assessment of plants with respect to their adaptation to environmental conditions and to interaction with other organisms. The proper observation of the phenotype decides about the quality of inference in both basic research (genomics, genetics, systems biology) and in applied studies (on plant resistance, agrotechnology, ecology), and also in breeding. The whole spectrum of methodology and research devoted to plant phenotypes is called "phenomics".
The development of biological sciences and plant breeding needs pose significant challenges in the field of phenomics, namely to the spectrum of interesting plant properties and the size of the populations studied. This stimulates the development of new measurement methods, changes in the way of conducting experiments and increasing research capacity. On the other hand, phenomics is more and more closely related to genomics, molecular biology and environmental research, which results from the need to interpret specific phenotypes against the background of the plant genotype and of the environment. All this means that tools with a high degree of technical sophistication are increasingly used for phenotyping of plants. New solutions are used to carry out experiments in controlled environmental conditions or in natural, but closely monitored conditions. Phenotyping is performed using methods that do not require the destruction of plants, which allows observation of developmental processes over time. Most importantly, thanks to the introduction of new devices that allow the detection of physico-chemical parameters, it is also possible to monitor processes that were escaping observation so far.
Due to the rapid technological progress, an increase in the costs of both construction and operation of plant phenotyping devices is observed. This applies to both experimental fields, which in accordance with modern requirements should be equipped with environmental monitoring systems, and some of them also with devices allowing simulation of biotic and abiotic stresses or phenotyping of root systems, as well as to greenhouses or breeding chambers, in which the desired conditions must be maintained regardless of the external environment. Both fields and closed installations are being equipped with phenotyping apparatus allowing to phenotype by means of imaging and remote sensing, sensors and systems of automatic treatment of plants with the required stress factors. The cost of installations required for modern plant phenotyping generally exceeds the capabilities of individual scientific units or enterprises. Establishment of the Network is aimed at conducting cooperation and coordination, which in the scale of the state will reduce the costs necessary to bear and increase the chance for Polish scientists and breeders to take advantage of innovative phenotyping methods.
The issue of development of plant research and plant phenotyping in particular is important both for basic research and for applied research aimed at providing plant products of adequate quality and in quantities expected by the state economy. The direct recipients of knowledge about plants are such sectors of economy as plant breeding and seed production; the indirect recipients are agriculture, food and biotechnology industry, environmental protection and engineering, landscape architecture, renewable energy production, pharmacy, construction and textile industry. Therefore, the operation of the proposed Network is in line with the assumptions of the National Strategy for Responsible Development until 2020 (with a view to 2030). It will be conducive to increasing the innovativeness of scientific research and knowledge-based development of many branches of the economy. It will also stimulate the progress in the field of robotization, sensorization, automation and digitization of research at various scales, from individual laboratories to nationwide experiments with economically important plants. It will force the development and implementation of IT solutions that provide the ability to automatically collect data and analyze and interpret them, including bioinformatic solutions for processing large data sets operating in the Internet environment. In the organizational sense, the Network will be conducive to the implementation of the Strategy's goals as a body that coordinates and optimizes scientific activities; it can also act as an advisory body.
In the sense of specific objectives of the above mentioned Strategy, the proposed project will be an important element of the state's support for agriculture and processing of agricultural products, including the production of high-quality food, as the sectors of the economy of key importance, both internal and external, due to a significant share in the state's revenues. The establishment of the Network favors the implementation of the objectives of KIS 4 "Innovative technologies, processes and products of the agri-food and forestry sector", in particular related to the support of creative plant farming using molecular and biotechnological tools. Phenomic approach also allows to take into account the biodiversity and adaptability of plants to changing environmental conditions, including climate change. It will accelerate the supply of modern and efficient tools to breeding companies that will support breeding new varieties, obtaining starting materials for breeding plants resistant to biotic factors (e.g. fungi or bacteria) and abiotic factors (such as drought, frost, soil salinity) and stimulation of key species breeding for Poland agriculture.
The assumed effects of the project implementation are:
- Modernization of the existing phenotyping infrastructure essential for the implementation of research and development projects responding to the challenges of scientific research in systems biology and areas of the economy that use plant materials.
- Construction of a new infrastructure allowing for undertaking projects that require "new generation" phenotyping - in strictly defined or precisely monitored environmental conditions, for the traits and features not yet observed.
- Respond to challenges defined in the Horizon 2020 framework program, in the area of "food security, sustainable agriculture", in the scope of Polish scientific community dealing with plants; the issue of plant phenotyping is included as one of the priorities of the framework program, which was reflected in the establishment of infrastructure priorities and creation of the leading ESFRI EMPHASIS platform.
2. Description of the unique nature of the project in terms of its national and international significance.
The progress in research on the impact of genetic and environmental factors on plant development can be made by developing new, non-invasive, precise, high-throughput phenotyping methods.
Currently, dynamic development of plant phenotyping techniques in greenhouse and field conditions is observed. The new methods overcome imperfections of previous techniques related to the measurement of plant phenotypic traits. The development of an efficient, repeatable and precise detection system of interesting biological changes at the plant's phenotype level is crucial for advances in broadly understood plant science. The aim is to provide the ability to conduct phenotypic assays at the scale and level of plant population research, which is very important when the parallel progress in high-throughput genome and transcriptome investigations occurs.
High-throughput, non-invasive plant phenotyping is associated with the use of various types of imaging (in the visible light spectrum, thermal, fluorescent, spectroscopic, 3D or using computed tomography), which must take place under strictly controlled environmental conditions. Modern greenhouses are currently dedicated to this purpose - platforms provide a full control of experimental conditions, as well as an efficient and collision-free course of the entire experiment. Phenotyping may involve the aboveground parts of the plant in relation to the dynamics of growth, morphology, anatomy, biomass, the degree of tissue damage resulted from stress factors (biotic and abiotic), physiology, as well as photosynthesis efficiency and many other properties. In addition, the developed systems enable imaging of the underground parts of the plant providing information on root system development. Dataset about the tested plants during the growing season could be collected at every stage of plant development avoiding its destruction, in contrast to traditional observational methods. Infrastructure for high-throughput phenotyping of aboveground parts of plants and roots have been created in many countries. Unfortunately, there is no investment of Polish research centers in this area.
Much attention is also paid to the development of mobile devices for imaging plants in natural (field) conditions and devices enabling precise monitoring of environmental conditions (including sensors for measuring atmospheric CO2, air and soil humidity, light intensity spectrum, temperature, wind speed and direction). In this way, field phenotyping platforms are created both to understand the processes occurring in the plant, as well as to determine the impact of the natural environment on these processes. These platforms must register the properties of growing plants without interfering with their environment. Their use in plant biology research provides a full picture of the morphological changes occurring throughout the growing season of plants. Observation of the so-called dynamic phenotypic traits is crucial in the analysis of vascular plant development processes, and in combination with the analysis of biochemical changes or metabolic processes can provide important basic knowledge. There are no such infrastructures in Poland, and progress in the field of genetics and plant breeding depends on the development of technologies that allow quick, efficient and at the same time non-invasive phenotyping of plants. In addition, it is possible to develop a full robotic breeding process (including fertilizer and water dosing, as well as temperature and air flow control).
Automatic phenotyping plays also a special role in the study of plant reactions to biotic stresses. While in the case of studying the impact of abiotic stresses on the crops physiology and development it is possible to control this process quite precisely, the interaction of crop plants with pathogens is more complex. Plant modifications during the infection depend on the developmental stages of pathogens, cycles of their annual and circadian development, degree of pathogenicity of isolates (usually subject to changes depending on the length of storage and passage of cultures on media prepared under laboratory conditions) as well as the plant development stage. Determination of the plant phenotype subjected to biotic stress is often subjective, which can be largely compensated by automatic phenotyping. An example may be the use of thermal and multi-vision or hyperspectral cameras as well as cameras using visible light. They allow monitoring the disease process, the speed of physiological changes and the development of symptoms of plant infection. Currently, such research can be conducted for a relatively small number of plants, which causes the subjectivity of conclusions (insufficient number of biological replicates) and the inability to study large populations. Automation of the phenotyping of plants subjected to stress is one of the main challenges of contemporary breeding work.
Environmental stresses cause a multifaceted plants reaction, both at the level of the entire plant (each plant organ, including root system and leaves), as well as the structural and functional level of the plant cell. The selective analysis of individual components of the plant's reaction to environmental factors is therefore insufficient and may lead to the generation of a fragmented model of the plant's functioning under environmental stress conditions. Different strategies of resistance/tolerance to stresses overlap and the need for simultaneous examination of them seems to be more justified. This is, among others, related to plants resistance/tolerance to drought, where simultaneous examination of the root system development and stomatal conductance (transpiration level) in the leaves and photosynthesis intensity on a large number of objects in monitored field conditions is crucial not only for the understanding of mechanisms of resistance, but also for the genotypes selection with desirable utility traits.
Modern phenotyping techniques are also important for basic research, e.g. in the field of system biology like monitoring photosynthetic activity, including gas exchange between the plant and the environment, water transport and cell growth and the development of each plant organ. Advanced imaging techniques allow three-dimensional reconstruction of tissues, plants and canopies. They include: X-ray tomography (e.g., for the root system assessment), multispectral fluorescence imaging (e.g., for photosynthesis monitoring), magnetic resonance imaging and nuclear magnetic resonance imaging (e.g., for in vivo metabolic changes monitoring). Such techniques are important, among others, to investigate the relationship between developmental plasticity and plant metabolome changes in the context of biotic and abiotic stress reactions conducted at IPG PAS.
The advantage of the proposed infrastructure is the possibility to initiate and escalate the cooperation of Polish scientists from many specialties, such as genetics and molecular biology, cytogenetics, physiology, phytopathology, proteomics and metabolomics, statistics and bioinformatics. Thanks to having specialists in many disciplines, as well as laboratory and advanced phenomics facilities, it will be possible to conduct comprehensive, modern research on the genetic basis of plant productivity and crop quality at the molecular, sub-cellular, cellular or the entire plant level. It also gives the opportunity to perform research on both basic and application aspects.
The research infrastructure discussed here is available in numerous EU countries, but many of the devices must be installed locally. Localization of infrastructure for field research is related to specific soil and climate conditions, unique in every place (environment), important for the study of genotype-environmental interaction. Experiments in controlled conditions should be carried out in places with both closed, automated growth chambers as well as greenhouses used to observe the full vegetative cycle. As far as the phenotyping platforms abroad are concerned, they are mostly fully used by national research and development programs or private companies that commission experiments. In addition, after 2021, when the EPPN2020 project will be completed, the European Commission will no longer finance international access to phenotyping installations.
3. Description of the applicant's institutional and personnel capacity.
Institute of Plant Genetic PAS, category "A" research institution, is a public sector research institute with a mission to carry out research on plant genetics and genomics, train highly-qualified scientists in this area, develop research methods and produce biological materials useful in breeding practice. The IPG PAS as a centre of agrobiology and molecular genetics, performs research on genetics, cytogenetics, genomics, proteomics, metabolomics, biotechnology, biometry and bioinformatics on crops and model plants (cereals, grasses, legumes, potato, oilseed rape, Arabidopsis thaliana or Medicago truncatula). The Institute comprises six departments: Environmental Stress Biology, Biometry and Bioinformatics, Biotechnology, Pathogen Genetics and Plant Resistance, Genomics, and Integrative Plant Biology (the latter created in 2014 on the basis of the EU FP7 ERA Chair BIO-TALENT project, GA 621321). Within departments, the work is organized into 15 flexible Teams. The research is financed from the statutory funds provided by the Ministry of Science and Higher Education as well as national and international competitive calls (National Science Centre, National Centre for Research and Development, Ministry of Agriculture and Rural Development, EU Programmes, ERA platforms; 49 research projects in total). The statutory research in 2017 and 2018 was divided into 6 topics: 1. Analysis of plant genomes and gene structures and functions; 2. Pathogen genetics and interactions with crop plants and antagonistic microorganisms; 3. Mechanisms of plant adaptation to environmental stresses; 4. Bioinformatics and statistical analysis of proteins and DNA; 5. Biotechnological tools for improvement of plant application potential; 6. Improvement of plant quality through modification of molecular and cellular regulatory mechanisms. The Institute employs 122 persons, including 25 experienced scientists (professors and assistant professors) and 52 young researchers (post docs and PhD students) as well as 45 technical and administrative staff. The quality of research activities, scientific plans, programmes and annual reports are guided and controlled by the Scientific Council, at present chaired by Prof. Zofia Szweykowska-Kulińska (Faculty of Biology, Adam Mickiewicz University in Poznań).
Laboratories of IPG PAS are equipped with all the basic facilities necessary for conducting research. This basic equipment includes various types of microscopes (including a cutting edge laser dissecting microscope), computers, hoods and laminar flow hoods, refrigerators and freezers, table centrifuges, electrophoresis sets, pH-meters, analytical balances, shakers, sterilisers, etc. The Institute has also some special facilities for common use, such as cold rooms and dark rooms, controlled environment chambers (a laboratory of 80 m2 with a complex of 5 controlled environment chambers), an isotope laboratory and equipment for water purification. Two liquid chromatography – mass spectrometry systems are installed. The first consists of a standard HPLC coupled to an ion trap low resolution mass spectrometer (Esquire, Bruker-Daltonics); this mass spectrometer may be equipped with electrospray (ESI) or chemical ionisation (APCI) ion sources so various classes of polar and non-polar metabolites may be analysed. The second one is a high resolution tandem mass spectrometer with Orbitrap ion analyser (Q-Exactive, Thermo) that is used together with either ultra-performance liquid chromatograph (Acquity, Waters) for metabolomic analyses or nano-UHPLC (Thermo) for proteomic applications. Phenotyping glasshouse experiments are performed in 2 complexes of glasshouses, with a total area of about 3200 m2. Two of the glasshouses are divided into small chambers (from 10 to 30 m2) and the remaining glasshouses are divided into big chambers (up to 170 m2). All but one glasshouse are equipped with tables or trolleys; one glasshouse is reserved for planting plants directly into the soil. The glasshouses are equipped with systems for the control of lighting and temperature. The lighting and air conditioning in the newest glasshouse with 6 chambers is digitally controlled. Seasonal experiments may also be performed in plastic tunnels. Such tunnels are located both at the IPG PAS premises in Poznan (total field area 1 ha) and in the experimental station located in Cerekwica (20 km away; total area of experimental fields 15 ha). Glasshouse experiments are performed with the help of field workers. The field group is equipped with all the basic tools and equipment plus chemical reagents for the superficial sterilisation of chambers and pest control. Despite careful management and maintenance, due to increasing number of research projects requiring increasingly complicated phenotyping, the current phenotyping facilities are not sufficient with respect to both throughput and technical level.
Potential and experience in the field of project coordination in which plant phenotyping occurs:
- POLAPGEN-BD (2010-2015) "Biotechnological tools for breeding cereals with increased resistance to drought" IPG PAS coordinator Paweł Krajewski, partners: 10 research institutions, 2 breeding companies (list of publications available at www.polapgen.pl).
- Multiannual Governmental Programme (2016-2020) "Increasing the use of domestic feed protein for the production of high quality animal products in conditions of sustainable development ", Area 2. New methods and techniques for improving the value of legume varieties, IPG PAS coordinator: W. Święcicki, Department of Genomics, consortium members: 4: 4 (University of Warmia and Mazury in Olsztyn, Nicolaus Copernicus University in Toruń, Strzelce Brreding Company, Institute of Plant Physiology PAS in Cracow)
- PBS3/A8/28/2015 SEGENMAS New generation sequencing and association mapping as methods for generating molecular markers of agricultural traits in narrow-leafed lupin, project coordinator B. Wolko (IPG PAS), consortium members: 7 (2 breeding companies, 2 PAS institutes, 3 universities), 1 March 2015 - 31 August 2018, Financing: NCBR.
Implementing national infrastructure projects
- 2017 – Application for maintaining research potential due to unexpected events (PLN 233 380) – greenhouses renovation
- 2016 – Application for restructuring funds (PLN 1 656 000) – for equipping a modern, interdisciplinary laboratory in the newly established Department of Integrative Plant Biology.
- 2014 – Application for restructuring funds (PLN 1 649 250) – for the establishment of Controlled Environment Chambers facility to work with in vitro cultures and to grow plants in the controlled conditions (temperature, light, humidity).
Implementing international infrastructure projects
- EU FP7 transPLANT Trans-national infrastructure for plant genomic science (2011-2015), project coordinated by EMBL-EBI. Development and implementation of recommendations and standards concerning plant phenotyping data and metadata.
- Horizon 2020 EPPN 2020 – European Plant Phenotyping Network 2020 (2017-2021), project coordinated by INRA. Participation in development of information systems for EPPN2020 and EMPHASIS projects.
- EU EMPHASIS-PREP, Support Group Member. Collaboration in preparation of the planned infrastructure.
National innovative research projects (over 2 mln PLN in total)
- Innovative Economy Programme 2007-2013, POLAPGEN-BD "Biotechnological tools for breeding cereals with increased resistance to drought" (coordinated by IPG PAS, total 23 400 000 PLN, approx. 6 000 000 for IPG PAS, 2010-2015).
- Multiannual Governmental Programme for 2016-2020 "Increasing the use of domestic feed proteins for high quality animal products under sustainable development" (33 936 000 PLN, 8 062 000 PLN for IPG PAS).
- NCBR SEGENMAS "New generation sequencing and association mapping as methods for generating molecular markers of agricultural traits in narrow-leafed lupin" (coordinated in IPG PAS, total 3 590 314,00 PLN, 1 486 057 for IPG PAS, 2015-2018).
- NCBR PBS BIOTRIGEN "Development and implementation of a model of wheat breeding based on biotechnological methods" (coordinated in IPG PAS, total 2 958 654 PLN, 1 708 978 for IPG PAS, 2013-2017).
- Ministry of Agriculture and Rural Development Projects: financing approved yearly, e.g. in 2017 10 projects, total financing 1 563 825 PLN for one year.
International innovative research projects (over 2 mln PLN in total)
- EU FP7, Pilot ERA Chair BIO-TALENT The Creation of the Department of Integrative Plant Biology (2014-2019), performed at IPG PAS, 1 935 839 Euro, additional financing from MNiSW 1 536 772 PLN.
- EU FP4 IMASCORE Integrated strategies for the management of stem canker in Europe (1997-2000)
- EU FP5 SECURE Stem canker of oilseed rape: molecular tools and mathematical modelling to deploy durable resistance (2002-2006)
- EU FP5 SAGES Sustainable Grasslands withstanding Environmental Stresses (2001-2003)
- EU FP7 LEGATO LEGumes for the Agriculture of Tomorrow (2014-2017)
- EU FP7 TRANSISTOR Trans-Cis elements regulating key switches in plant development (2005-2009)
- EU FP7 SYSFLO Training in systems biology applied to flowering (2009-20013)
- EU FP7 ITN EPITRAITS Epigenetic regulation of economically important plant traits (2012-2016).
- ERA-CAPS FLOWPLAST Plasticity of flowering time in response to environmental signals in Arabidopsis thaliana (2014-2017).
Main publications
(categorized by the department/research field)
Environmental Stress Biology
- Perlikowski D., Kierszniowska S., Sawikowska A., Krajewski P., Rapacz M., Eckhardt Ä., Kosmala A. (2016). Remodeling of leaf cellular glycerolipid composition under drought and re-hydration conditions in grasses from the Lolium-Festuca complex. Frontiers in Plant Science 7:1027.
- Perlikowski D., Czyżniejewski M., Marczak Ł., Augustyniak A., Kosmala A. (2016). Water deficit affects primary metabolism differently in two Lolium multiflorum/Festuca arundinacea introgression forms with a distinct capacity for photosynthesis and membrane regeneration. Frontiers in Plant Science 7:1063.
- Kosmala A., Perlikowski D., Pawłowicz I, Rapacz M. (2012). Changes in the chloroplast proteome following water deficit and subsequent watering in a high and a low drought tolerant genotype of Festuca arundinacea. Journal of Experimental Botany 63: 6161-6172.
- Kosmala A., Bocian A., Rapacz M., Jurczyk B., Zwierzykowski Z. (2009). Identification of leaf proteins differentially accumulated during cold acclimation between Festuca pratensis plants with distinct levels of frost tolerance. Journal of Experimental Botany 60: 3595-3609.
- Kosmala A., Zwierzykowski Z., Gąsior D., Rapacz M., Zwierzykowska E., Humphreys M.W. (2006). GISH/FISH mapping of genes for freezing tolerance transferred from Festuca pratensis to Lolium multiflorum. Heredity 96: 243-251.
Biotechnology
- Kapusta J., Modelska A., Figlerowicz M., Pniewski T., Letellier M., Lisowa O., Yusibov V., Koprowski H., Płucienniczak A., Legocki A.B. (1999). A plant-derived edible vaccine against hepatitis B virus. FASEB J, 13:1796–1799.
- Kostrzak A., Cervantes Gonzalez M., Guetard D., Nagaraju D.B., Wain-Hobson S., Tepfer D., Pniewski T., Sala M. (2009). Oral administration of low doses of plant-based HbsAg induced antigen-specific IgAs and IgGs in mice, without increasing levels of regulatory T cells. Vaccine, 27:4798-4807.
- Pniewski T., Kapusta J., Bociąg P., Wojciechowicz J., Kostrzak A., Gdula M., Fedorowicz-Strońska O., Wójcik P., Otta H., Samardakiewicz S., Wolko B., Płucienniczak A. (2011). Low-dose oral immunization with lyophilized tissue of herbicide-resistant lettuce expressing hepatitis B surface antigen for prototype plant-derived vaccine tablet formulation. J Appl Genet, 52:125-136.
- Pniewski T., Kapusta J., Bociąg P., Kostrzak A., Fedorowicz-Strońska O., Czyż M., Gdula M., Krajewski P., Wolko B., Płucienniczak A. (2012). Plant expression, lyophilisation and storage of HBV medium and large surface antigens for a prototype oral vaccine formulation. Plant Cell Rep, 31:585-595.
- Pyrski M., Rugowska A., Wierzbiński K.R., Kasprzyk A., Bogusiewicz M., Bociąg P., Samardakiewicz S., Czyż M., Kurpisz M., Pniewski T. (2017). HbcAg produced in transgenic tobacco triggers Th1 and Th2 response when intramuscularly delivered. Vaccine, 35: 5714–5721.
Pathogen Genetics and Plant Resistance
- Piasecka A., Sawikowska A., Kuczyńska A., Ogrodowicz P., Mikołajczak K., Krystkowiak K., Gudyś K., Guzy-Wróbelska J., Krajewski P., Kachlicki P. (2017). Drought related secondary metabolites of barley (Hordeum vulgare L.) leaves and their mQTLs. The Plant Journal 89: 898-913.
- Piasecka A., Sawikowska A., Krajewski P., Kachlicki P. (2015). Combined mass spectrometric and chromatographic methods for in-depth analysis of phenolic secondary metabolites in barley leaves. J. Mass Spectrom. 50: 513-532.
- Wojakowska A., Muth D., Narożna D., Mądrzak C., Stobiecki M., Kachlicki P. (2013). Changes of phenolic secondary metabolite profiles in the reaction of narrow leaf lupin (Lupinus angustifolius) plants to infections with Colletotrichum lupini fungus or treatment with its toxin. Metabolomics 9: 575-589.
- Kachlicki P., Einhorn J., Muth D., Kerhoas L., Stobiecki M. (2008). Evaluation of glycosylation and malonylation patterns in flavonoid glycosides during lc/ms/ms metabolite profiling J. Mass Spectrom. 43: 572-586.
- Kachlicki P., Marczak Ł., Kerhoas L., Einhorn J., Stobiecki M. (2005). Profiling isoflavone conjugates in root extracts of lupine species with LC/ESI/MSn systems. J. Mass Spectrom. 40: 1088-1103.
Genomics
- Nelson M.N., Książkiewicz M., Rychel S., Besharat N., Taylor C., Wyrwa K., Jost R., Erskine W., Cowling W.A., Berger J.D., Batley J., Weller J.L., Naganowska B., Wolko B. (2017). The loss of vernalization requirement in narrow-leafed lupin is associated with a deletion in the promoter and de-repressed expression of a Flowering Locus T (FT) homologue. New Phytologist 213: 220–232. DOI: 10.1111/nph.14094.
- Susek K., Bielski W., Hasterok R., Naganowska B., Wolko B. (2016). A first glimpse of wild lupin karyotype variation as revealed by comparative cytogenetic mapping. Frontiers in Plant Science 7:1152. DOI 10.3389/fpls.2016.01152.
- Książkiewicz M., Rychel S., Nelson M.N., Wyrwa K., Naganowska B., Wolko B. (2016). Expansion of the phosphatidylethanolamine binding protein family in legumes: a case study of Lupinus angustifolius L. FLOWERING LOCUS T homologs, LanFTc1 and LanFTc2. BMC Genomics 17:820. DOI 10.1186/s12864-016-3150-z.
- Wyrwa K., Książkiewicz M., Szczepaniak A., Susek K., Podkowiński J., Naganowska B. (2016). Integration of Lupinus angustifolius L. (narrow-leafed lupin) genome maps and comparative mapping within legumes. Chromosome Research 24 (3): 355–378.
- Przysiecka Ł., Książkiewicz M., Wolko B., Naganowska B. (2015). Structure, expression profile and phylogenetic inference of chalcone isomerase-like genes from the narrow-leafed lupin (Lupinus angustifolius L.) genome. Frontiers in Plant Science 6:268.
Biometry and Bioinformatics
- You Y., Sawikowska A., Neumann M., Pose D., Capovilla G., Langenecker T., Neher R.A., Krajewski P. Schmid M. (2017). Temporal dynamics of gene expression and histone marks at the Arabidopsis shoot meristem during flowering. Nature Communication 8: 15120.
- Ćwiek-Kupczyńska H., Altmann T., Arend D., Arnaud E., Chen D., Cornut G., Fiorani F., Frohmberg W., Junker A., Klukas Ch., Lange M., Mazurek C., Nafissi A., Neveu P., van Oeveren J., Pommier C., Poorter H., Rocca-Serra Ph., Sansone S-A., Scholz U., van Schriek M., Seren Ű., Usadel B., Weise S., Kersey P., Krajewski P. (2016). Measures for interoperability of phenotypic data: minimum information requirements and formatting. Plant Methods 12:44. DOI: 10.1186/s13007-016-0144-4.
- Krajewski P., Chen D., Ćwiek H., van Dijk A.D.J., Fiorani F., Kersey P., Klukas C., Lange M., Markiewicz A., Nap J.P., van Oeveren J., Pommier C., Scholz U., van Schriek M., Usadel B., Weise S. (2015). Towards recommendations for metadata and data handling in plant phenotyping. J. Exp. Bot. 66 (18): 5417-5427.
- Bailey T., Krajewski P., Ladunga I., Lefebvre C., Li Q., Liu T., Madrigal P., Taslim C., Zhang J. (2013). Practical guidelines for the comprehensive analysis of ChIP-seq data. PloS Computational Biology 9: e1003326.
- Kaufmann K., Wellmer F., Muiño J.M., Ferrier T., Wuest S., Kumar V., Serrano-Mislata A., Madueño F., Krajewski P., Meyerowitz E.M., Angenent G.C., Riechmann J.L. (2010). Orchestration of floral initiation by APETALA1. Science 328: 85-9.
Integrative Plant Biology
- Malinowski R, Novák O, Borhan MH, Spíchal L, Strnad M, Rolfe SA (2016) The role of cytokinins in clubroot disease. Eur J Plant Pathol 145: 543-557.
- Rolfe S.A., Strelkov S., Links M., Clarke W.E., Robinson S., Djavaheri M., Malinowski R., Haddadi P., Kagale S., Parkin I., Taheri A., Borhan M.H. (2016). The compact genome of the plant pathogen Plasmodiophora brassicae is adapted to intracellular interactions with host Brassica spp. BMC Genomics. 17 (1)
- Malinowski R., Smith JA., Fleming AJ., Scholes JD., Rolfe SA. (2012). Gall formation in clubroot-infected Arabidopsis results from an increase in existing meristematic activities of the host but is not essential for the completion of the pathogen life cycle. The Plant Journal 71: 226-238.
- Kuwabara A., Backhaus A., Malinowski R., Bauch M., Hunt L., Nagata T., Monk N., Sanguinetti G., Fleming A.J. (2011). A shift towards smaller cell size via manipulation of cell cycle gene expression acts to smoothen Arabidopsis leaf shape. Plant Physiology 156: 2196-21206.
- Malinowski R. Kasprzewska A., Fleming A.J. (2011). Targeted manipulation of leaf form via local growth repression. The Plant Journal 66: 941-952.
Patents
- Transgenic plant cells producing HBcAg antigen protein and their application in the process of vaccine production, no 216099.
- Transgenic plant cells producing M-HBsAg antigen protein and their application in the process of vaccine production, no 217229.
- Transgenic plant cells producing L-HBsAg antigen protein and their application in the process of vaccine production, no 217212.
- Universal carrier of bacterial-derived antigen, anti-microbial vaccine, the production of universal antigen carrier, application of a universal antigen carrier, no P.393507.
- Expression cassette, T-DNA particle, plant expression vector, transgenic plant cell and their application for the production of a vaccine, no P 382769.
- Method of genetic modification of plants, especially potatoes, no P.360191.
- Co-authorship of crop cultivars (pea, grasses).
4. Description of the level of interest in the project shown by the scientific and research community and the relevant businesses at national and international level, in particular during the operational phase of the proposed research infrastructure.
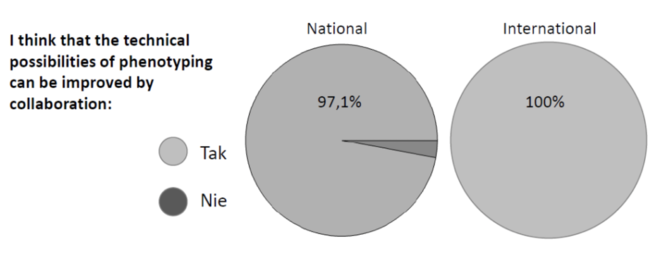
The challenges of the Polish scientific community in improvement of the phenotyping capacities are known to proposers from numerous contacts and collaborative projects. Also, in 2017 IPG PAS carried out a questionnaire on plant phenotyping in Poland. 32 responses were received from universities (37%), seed and breeding companies (31%), PAS institutes (20%), state institutes (10%) and other units. Among the answers to many questions, the vast majority of respondents identified their phenotyping methods as mostly traditional (91%); some respondents showed the use of image-based methods (28%, a number of them being the units that used access to infrastructure as part of the EU project FP7 EPPN). The most urgent needs for improvement were indicated in the area of automatic phenotyping under natural conditions (69%), under controlled conditions (47%) and in phytotrons (34%). Answers to the question regarding the importance of national and international cooperation are presented in Fig.
Letters of intent supporting the initiative of creating the Network and describing the motivations of individual units were sent by: Institute of Plant Physiology PAS; Institute of Biochemistry and Biophysics PAS; Department of Chemistry, Technical University Rzeszów; Departmet of Genetics and Breeding, Poznań Life Sciences University; Department of Genetics, Breeding and Biotechnology, Warsaw University of Life Sciences; Institute of Breeding and Acclimatization, State Research Institute; Institute of Soil Science and Cultivation, State Research Institute; Departmet of Plant Breeding and Seed Production, Wrocław University of Life Sciences; Danko Plant Breeders Ltd., Poznań Plant Breeders Ltd.
After the creation of the Network, its infrastructure will be open to conducting research by all entities accepting the access rules adopted by the partners. In the case of Poland's accession to the ESFRI EMPHASIS platform, this infrastructure will enter the European plant phenotyping system and will also be used, on agreed terms, by foreign researchers.
The network management structure will include the participation of foreign experts in the planning and periodic evaluation of the activity and the participation of an observer on behalf of EMPHASIS.
5. Estimated project implementation costs, including those that are to be incurred during the operational phase of the proposed research infrastructure, as well as information regarding the proposed funding sources.
The cost of the current infrastructure (with the possibility of its development)
Annual average costs of plant phenotyping in IGR PAN evaluated on the basis of data for 2016-2017 are presented in Table
|
Running costs (1 year) |
Cerekwica |
CUR |
Greenhouses and fields in Poznań |
Total |
|
Depreciation |
12 031,20 |
412 312,80 |
318 389,88 |
|
|
Salary costs |
149 097,32 |
50 734,64 |
133 783,24 |
|
|
Electric energy |
13 976,51 |
56 000,00 |
88 800,00 |
|
|
Garbage |
17 229,51 |
|||
|
Water |
968,45 |
853,47 |
5 768,00 |
|
|
Gas |
150 360,89 |
|||
|
Materials and services |
27 682,03 |
1 276,53 |
149 806,56 |
|
|
Total |
203 755,51 |
521 177,44 |
864 138,08 |
1 589 071,03 |
|
Distribution of salary costs by sources of financing |
||||
|
Statutory subsidy |
135 076,14 |
35 241,02 |
115 214,87 |
|
|
Projects (direct and indirect costs) |
14 021,18 |
15 493,62 |
18 568,37 |
|
|
Breakdown of other costs by sources of financing |
||||
|
Statutory subsidy |
43 726,55 |
376 354,24 |
584 283,87 |
|
|
Projects (direct and indirect costs) |
10 931,64 |
94 088,56 |
146 070,97 |
|
|
Total subsidy |
178 802,69 |
411 595,26 |
699 498,74 |
1 289 896,69 |
|
Total projects |
24 952,82 |
109 582,18 |
164 639,34 |
299 174,34 |
It is planned to retain a similar structure of maintenance costs and their coverage for the developed and retrofitted infrastructure of IPG PAS during the Network's operation phase. It is planned to apply (with other Network’s partners, within the framework of established principles of complementarity) for competitive equipment funds to cover the costs of innovations in the phenotyping systems (environmental monitoring systems, root phenotyping systems), with own contribution resulting from depreciation. The increase in costs of running the infrastructure resulting from the application of new technologies will be covered from the direct costs of research projects (purchase of materials).
Construction of regional clusters for phenotyping under controlled conditions
It is planned to apply for funds for the construction of 2-3 regional clusters for the phenotyping of plants with an area of about 1000 m2 each, consisting of greenhouses of about 350 m2 (4 greenhouse cameras 58 m2, technological part 30 m2), steel-aluminum structure, equipped with climate control (in the case of IPG PAS coupled with phytotron controls), energy-saving curtains, ventilation control, mixing fans, humidification or air cooling with water mist, general remote control via the Internet, sliding systems, mobile cultivation tables and gutters, collecting rainwater from the roof of the greenhouse and using it after mechanical filtration for the purposes of sprinklers, and watering and fertilizing plants. The basic cost of one such greenhouse is estimated at PLN 3.7 million; the cost of equipment that automates phenotyping and material manipulation depends on additional specifications tailored to the specific needs of the cluster or its element. The planned financing is: own funds 20%, funds from the state budget 60%, funds from local budgets 10%, other funds, including private ones, 10%. The location of clusters and their exact technical specification will be selected based on the analysis of partners' needs and joint decisions of the Network. The construction and use costs of the installation will include appropriate amounts for IT infrastructure and services.
Construction of central infrastructure for highly automated plant phenotyping
The location of the central infrastructure (IC) will be selected on the basis of the analysis of the possibilities and needs of the partners, using the existing infrastructure as much as possible. The investment will consist of an ultra-modern greenhouse, in which automatic control systems for environmental conditions, plant cultivation systems and sets of cameras operating in various light ranges will be installed, allowing for permanent monitoring of plant growth and development, and measurements of selected physiological and yielding parameters. The collected data will be processed by the specially created IT unit equipped with the appropriate hardware and software. The planning, construction and commissioning of the IC will be completed within three years (preparation of documentation and construction - years 1-2, equipment fitting, testing and calibration of procedures - year 3). The implementation of the investment is planned to be financed, depending on the availability of financial resources, from the state budget funds granted in a competitive manner, with limited own contribution of the Network's partners. IC operational costs (service, maintenance, media, experience) will be financed from projects carrying out research. These will be projects financed from funds for statutory research of these units and from research projects obtained in national (NCN, NCBiR) and international calls (Horizon 2020); the share of breeding companies in such projects is usually associated with companies supplying their own contribution. With the planned capacity of 1200 plants per experiment, the cost of conducting the experiment in the basic version (control variant) will amount to approximately PLN 370 per one plant (pot). The IC construction cost will amount to approx. PLN 34 million (including: construction of a greenhouse of 8 million, cropping and plant measurement systems, imaging systems, plant transport system, IT systems – 20 million, a building with the accompanying equipment of 800 m2 – 6 million); operating costs are about PLN 1.5 million annually (including: the cost of maintaining a greenhouse of 0.20, building 0.15, remuneration 0.70, consumables 0.20, cost of growing plants 0.25).
6. Description of the scientific and technical concept underlying the project implementation.
The scientific and technical concept of the Network is based on the assumption that the introduction of modern methods based on innovative technical solutions to plant phenotyping will be easier and cheaper in the environment of cooperating scientific and industrial units. The network will use both the existing infrastructure, i.e., the objects currently located at its partners, as well as newly built ones. Proper, modern phenotyping of plants requires conducting experiments both in highly specialized "central" platforms operating at the national or regional level, as well as in distributed installations managed by individual partners. The Network action plan includes four key elements described below. It should be noted that the implementation of tasks will depend on the financial resources obtained, but even the fulfillment of the basic elements (listed in items 1 and 2) with limited funding justifies setting up the Network.
- Cooperation in maintaining, modernizing and standardizing existing infrastructure for plant phenotyping located at partners.
- Construction of a common infrastructure for phenotyping under controlled conditions jointly managed by partners operating in one region.
- The construction of central infrastructure for highly automated phenotyping that works for the whole community.
- Cooperation in the standardization of approaches to annotation, storage and analysis of phenotypic data.
Ad 1. The network will organize training on plant phenotyping and exchange of information on the existing infrastructure of individual partners (greenhouses, phytotrons, experimental fields, measuring equipment). The aim of these activities will be to introduce innovations to the activities of individual partners, achieve a higher level of standardization and improve the complementarity of the apparatus at the regional and national level. It will allow to undertake joint research projects requiring different phenotyping technologies (e.g., phenotyping in terms of anatomical features and characteristics related to productivity), phenotyping at various levels of plant organization (e.g., at the level of tissues and entire plants), or phenotyping with simultaneous observation of biochemical characteristics (profiling of the proteome or metabolome). Cooperation of units will also concern conducting series of experiments in various environmental conditions, in experimental fields equipped with compatible systems of observation of plant and canopy features using mobile and remote sensing devices, root phenotyping systems and systems for monitoring weather and soil parameters. Thanks to the cooperation it will be possible to share particularly expensive equipment (e.g., chromatographs and mass spectrometers, microscopes or mobile devices). The activities will also allow to optimize financing of the phenomics at the national scale through the elaboration of priorities. The subject of modern phenomics will be introduced into doctoral programs run by partners, which will increase the scientific level of research, doctoral dissertations and habilitations.
Ad 2. The construction of new infrastructure for precise phenotyping of plants under strictly controlled conditions (phytotrons, growth chambers, greenhouses) with the capacity appropriate for the study of segregating populations or variety panels is a prerequisite for the proper conduct of modern research on comparing different plant genotypes with respect to the basic functions of their systems and plant reactions to changing environmental conditions, including biotic and abiotic stresses. Due to the significant cost of construction and use, such infrastructure should be grouped in regional clusters, which will allow its optimal use and application of common power and control installations. It is also important to group installations of various uses on one site (e.g., phytotrons to study stress responses and greenhouses connected with them to study yield components).
Ad 3. Non-invasive phenotyping under controlled conditions and during plant development, using the latest technologies of imaging in various ranges of light waves, allows to learn the course of processes taking place in plants during the reaction to biotic or abiotic stress. Platforms allowing this type of observation are equipped with sets of cameras operating in different wave ranges and with devices for automatic transfer of plants or cameras to observation points and to control / change conditions (e.g., automatic maintenance of the desired, varying soil moisture levels). Such infrastructure should be used in projects of particular significance for the progress of research on plants in the country (e.g., association studies using a wide representation of crop varieties of selected species) and, due to the high construction and maintenance costs, managed by the representation of a broad scientific community.
Ad 4. The development of phenotyping technologies necessitates proper progress in the field of information systems controlling the infrastructure and allowing for proper collection and interpretation of results. The Network will develop protocols that use the latest IT knowledge and semantic techniques used in European infrastructures. Detailed tasks in the field of IT systems development will be formulated depending on the progress of building the infrastructure.
The Network's work in the organization and operation phase will be carried out according to the following schedule:
- Organizational phase: defining the objectives accepted by the partners, developing rules for cooperation, defining the infrastructure potential of the partners, defining the network management methods (Partners' Council, rotating chairmanship and technical coordination), developing a business plan, milestone – memorandum of understanding (December 2018); elaboration of formal and legal aspects of the agreement, milestone – signing of the agreement on the establishment of the Network (June 2019).
- Operation phase, national level: identification of research topics requiring retrofitting and sharing of existing infrastructure, applying for appropriate funds in competitive calls, milestone – submitted min. 2 joint research and development or infrastructure projects (December 2019); elaboration of detailed location and technical assumptions for the newly built infrastructure, applying for funds in competitive or other calls, milestone – submission of applications for financing of the construction of common infrastructure (June 2020).
- Operation phase, international level: development of principles for the representation of the Network and the principles of cooperation with European infrastructures, milestone – submission of an application for Poland's accession to the ESFRI EMPHASIS infrastructure (June 2020); integration of the national infrastructure with the EMPHASIS platform, milestone – organization of the ESFRI EMPHASIS scientific conference in Poland (2021).
During the implementation of the above schedule, the following risks should be considered:
- Disruption of organizational work and operation of the Network through the competitive behavior of individuals. This threat will be reduced by maintaining full transparency of goals and principles of operation from the beginning of the process, which will result in joining the agreement only by entities accepting its goals and principles. When developing documents and project applications, specific and mutually complementing partner specializations will be taken into account. It should be assumed that competition is a natural element of scientific activity, and the Network will strive to ensure that joint ventures implement the ambitions of partners. Experiences from major projects in Poland (e.g., POLAPGEN-BD, GENSEC, SEGENMAS, HYBRE, BIOTRIGEN) and experience from other countries (e.g., Belgium, the Netherlands, Germany) show that properly planned joint actions change the viewpoint from a strictly "competitive" to "willingness to cooperate".
- Problems with defining and adhering to the principles of infrastructure sharing. In addition to the full transparency of the Network agreements and activities, the threat will be reduced by the work of the team coordinating the use of any common infrastructure being in constant contact and running a schedule of experiments available online.
- Insufficient staffing of the modernized and new infrastructure with personnel responsible for its functioning. The risk will be reduced by a realistic assessment of the capabilities of existing personnel and research staff at the cost calculation stage.
- Problems with the processing and integration of experimental data. The threat will be reduced by establishing local data curators (in units or clusters of installations) responsible for cooperation within the Network in accordance with the developed principles. In addition, the developed tools will allow the possibility of distributed data storage and processing.
7. Description of the conceptual framework for international cooperation on project implementation.
One of the objectives of establishing the Network is to include the national infrastructure in the EMPHASIS platform, which was established by ESFRI for the development of methods and applications of plant phenotyping as a priority in the European Research Area, with a view to securing food in a changing climate. The main goals of EMPHASIS are the organization and support of the operation of national plant phenotyping platforms and the organization of their cooperation. EMPHASIS preparatory phase – EMPHASIS-PREP project – is in progress until 2020, aimed at the progress of national infrastructures to the level of legal, financial and technical organization appropriate for the commencement of coordinated action. This phase is supported by the Horizon 2020 EPPN2020 project, funded in the years 2017-2021, devoted to the issues of technological and organizational development of phenotyping under controlled conditions; the project also finances the access of research groups to existing phenotyping installations by funding the implementation of experiments on a competitive basis. Cooperation with the EMPHASIS platform will be an indispensable element of the development of phenomics and overall plant research in Poland, ensuring both exchange of knowledge with European partners and the sharing of national and foreign infrastructure with them.
The IPG PAS is present in the structures leading to the EMPHASIS operating phase as a partner in the EPPN2020 project (JRA3 package "Building a consistent information system and defining standardization strategies") and a Support Group member of EMPHASIS-PREP project (the group units that expressed support for the EMPHASIS objectives by signing the document "EMPHASIS-PREP: Policy Manifesto". IPG PAS actively participates in EMPHASIS meetings, including Plant Phenotyping Forum, 22-24 November 2017, Tartu, Estonia, and in the Support Groups Meetings (22-23.03.2018, Jülich, Germany). The letter supporting the Polish Network initiative was issued by the EMPHASIS coordinator prof. Ulrich Schurr, Forschungszentrum Jülich.
The merit of the present activity of IPG PAS under EPPN2020 and EMPHASIS concerns work on the methods of collection and annotation of phenotypic data necessary for the implementation of information systems. These works were initiated in 2011-2015 by the participation of the Biometrics and Bioinformatics Department of IPG PAS in the EU FP7 TransPlant infrastructural project developing systems supporting plant research; in that project, the Polish group coordinated the development of the Minimum Information About Plant Phenotyping Experiment (MIAPPE; www.miappe.org) recommendations described in
8. Other relevant information necessary to evaluate the application
-
9. Full name, phone number and e-mail address of the person responsible for drafting the application.
prof. dr hab. Paweł Krajewski, +48 616550238, pkra@igr.poznan.pl
Date of preparation
11 / 06 / 2018
Funding program
Polish Roadmap for Research Infrastructures
References
- Measures for interoperability of phenotypic data: minimum information requirements and formatting.Springer Nature. https://doi.org/10.1186/s13007-016-0144-4
- Towards recommendations for metadata and data handling in plant phenotyping. https://doi.org/10.1093/jxb/erv271