|
Research Ideas and Outcomes :
Research Idea
|
|
Corresponding author: Shantibhusan Senapati (senapati@ils.res.in)
Received: 27 May 2020 | Published: 29 May 2020
© 2020 Shantibhusan Senapati, Jayalaxmi Dash, Manisha Sethi, Subhankar Chakraborty
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation: Senapati S, Dash J, Sethi M, Chakraborty S (2020) Bioengineered probiotics to control SARS-CoV-2 infection. Research Ideas and Outcomes 6: e54802. https://doi.org/10.3897/rio.6.e54802
|
|
Abstract
The outbreak of 2019 novel corona virus disease (COVID-19) is now a global public health crisis and declared as a pandemic. Several recent studies suggest that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein binds to human angiotensin-converting enzyme 2 (ACE2). The information obtained from these structural and biochemical studies provides a strong rationale to target SARS-CoV-2 spike protein and ACE2 interaction for developing therapeutics against this viral infection. Here, we propose to discuss the scope of bioengineered probiotics expressing human ACE2 as a novel therapeutic to control the viral outbreak.
Keywords
SARS-CoV-2, ACE2, probiotics
Overview and background
In a recent study,
ACE2 is mainly expressed by epithelial cells of intestine, lung, kidney, and blood vessels. Although, lung is the major organ where SARS-CoV-2 associated pathology is more common and severe, some patients also manifest gastrointestinal symptoms like diarrhea (
Probiotics generally regarded as safe (GRAS), are known to control multiple gut associated illness with almost no side effects. World health organization (WHO) has defined probiotics as “live microorganisms which when administered in adequate amounts confer a health benefit on the host”. In recent years, bioengineering of probiotic organisms has opened a wide range of opportunities to use these organisms as delivery vehicles, which finds application in immunomodulation, drug and vaccine delivery. Knowledge of different microbes or their toxins binding to host receptors for their pathogenesis have encouraged researchers to engineer probiotics expressing host receptor or toxin receptor mimics (
Objectives
To design probiotics for controling SARS-CoV-2 infection or transmission
Implementation
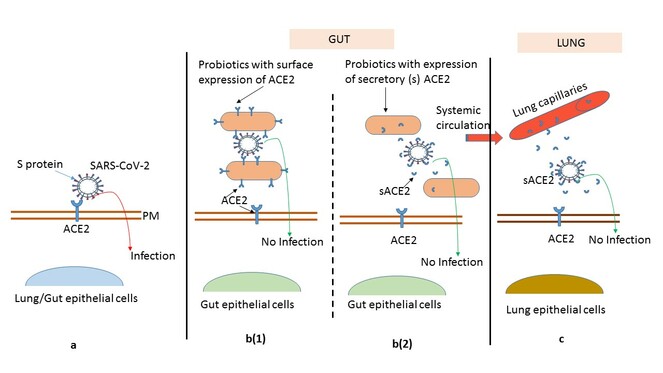
Based on the concept of probiotics as receptor mimics, engineered probiotics expressing ACE2 could be potential bio-remedies to neutralize SARS-CoV-2.
Bioengineered probiotics to control SARS-CoV-2 infection. (a) Pathogenesis of SARS-CoV-2 depends on interaction between S protein of virus and angiotensin-converting enzyme 2 (ACE2) expressed on the surface of host cells. (b (1)) Engineered probiotics with expression of cell bound ACE2, sequesters the virus by making it bind to the ACE2 receptor on its surface thus inhibiting the viral entry into gut epithelial cells. (b (2)) The secreted form of ACE2 (sACE2) produced by probiotics confiscates the virus by binding to S proteins and masking their binding sites for gut epithelial ACE2. (c) The sACE2 could also have systemic effects due to its absorption into circulation and inhibiting the virus binding at distant organs like lungs.
Acknowledgements
SS and JD thank the Department of Biotechnology, Govt. of India for supporting ongoing grant on probiotics (BT/ILS/Flagship/2019). SS also thanks director, ILS Bhubaneswar for supporting the reserach on probiotics and coronavirus. We thank Dr. Neera Singh, ProCyto Labs Pvt. Ltd., India for editing the manuscript.
Author contributions
SS conceived the idea; SS, JD, MS and SC drafted the article.
Conflicts of interest
No potential conflict of interest declared.
References
-
Diarrhoea may be underestimated: a missing link in 2019 novel coronavirus.Gut2020‑320832. https://doi.org/10.1136/gutjnl-2020-320832
-
Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2.Cell181:905‑91. https://doi.org/10.1016/j.cell.2020.04.004
-
Engineered probiotics: Applications and biological containment.Annual Review of Food Science and Technology8(1):353‑370. https://doi.org/10.1146/annurev-food-030216-030256
-
Expression of human ACE2 in Lactobacillus and beneficial effects in diabetic retinopathy in mice.Molecular Therapy - Methods & Clinical Development14:161‑170. https://doi.org/10.1016/j.omtm.2019.06.007
-
Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein.Cellhttps://doi.org/10.1016/j.cell.2020.02.058
-
Structural and functional basis of SARS-CoV-2 entry by using 2 human ACE2.Cellhttps://doi.org/10.1016/j.cell.2020.03.045