|
Research Ideas and Outcomes :
Methods
|
|
Corresponding author: Nicolas-George Homer Eliades (niceliades@gmail.com)
Received: 06 Apr 2020 | Published: 08 Apr 2020
© 2020 Constantinos Pericleous, Nicolas-George Eliades
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation: Pericleous C, Eliades N-GH (2020) An approach for the mass propagation of Cupressus sempervirens L. (Cupressaceae), for quality propagule production. Research Ideas and Outcomes 6: e52947. https://doi.org/10.3897/rio.6.e52947
|
|
Abstract
The scenarios of climate change in Mediterranean regions bring back the need for tree species tolerant to drought and disease, for agro- and urban-forestry purposes, landscape rehabilitation, as well as for post-fire and quarry restoration plans. Therefore, forest industries focus on selecting, propagating and growing superior trees. The Cupressus sempervirens L. is such a coniferous tree species, with a fundamental ecological, financial and ornamental role in the Mediterranean region. The aim of this study was to develop an efficient macropropagation protocol, which would lead to mass-selected genotypes or phenotypes of C. sempervirens f. sempervirens. For this purpose the axillary shoot multiplication method was used, by adopting both cutting propagation and intermittent mist methods. These methods were used for the first time in the macropropagation of C. sempervirens f. sempervirens. For shoot proliferation, 24 different modified macropropagation treatments at different concentrations and combinations were tested: shoot cuttings, concentrations of K-IBA and rooting systems. The elongation and root induction was carried out in an intermittent mist system for 50 days, then the new plantlets were moved for acclimatization for 15 days in a greenhouse. The current workflow presents an effective preliminary protocol with clear steps and treatments for macropropagation of C. sempervirens f. sempervirens. The developed protocol for macropropagation of tree species ensures cost- and time-efficient propagation. Produced plantlets were developed efficiently under in vivo conditions, allowing to propagate and store genetic material for conservation and domestication. This protocol can be generated for other tree species of the Cupressus genus and the Cupressaceae family.
Keywords
Cupressaceae, K-IBA, Mediterranean cypress, macropropagation, mist system, plant propagation, woody trees
Introduction
Propagation techniques for woody trees are used for producing plantlets and imply the culture of aseptic small sections of tissues and organs in vessels with defined culture and under controlled environmental conditions. These techniques have become an increasingly important tool for both science and commercial application in recent years (
The success of propagation involves several factors, such as the composition of the culture medium, culture environment, and genotype (
The scenarios of climate change in Mediterranean regions bring back the need for the preference of tree species tolerant to drought and disease, for both timber production and for urban forestry purposes. The Cupressus sempervirens L. (Mediterranean cypress) is a coniferous tree species, which has a fundamental ecological, financial and ornamental role in the Mediterranean region (
As regards to the C. sempervirens timber, it has interesting characteristics of high natural durability and straightness. From ancient times its wood has been particularly appreciated for its resistance to fungi and insects, especially if immersed in water (
Since the 1970s, and after the introduction of the serious disease of the “Cypress canker” in Europe (most likely from the USA), which is caused by the Seiridium cardinal fungus, recorded over large Mediterranean areas and leading to damage in forests, nurseries and ornamental plantations, the disease has become a limiting factor to cypress planting. Fungus management requires the removal of infested trees and the use of genetically resistant forms, and hence, clones have been recently patented (clonal selection in natural stands where the disease was present or by cross-breeding between selected trees in experimental fields) (
Although a number of reports have indicated that propagation can be successfully applied to Cupressus species (
Materials and Method
Sampling of shoots
For sampling of plant materials, the general guidelines for plant propagation need to be adopted (e.g.
Experiment design
Methodology
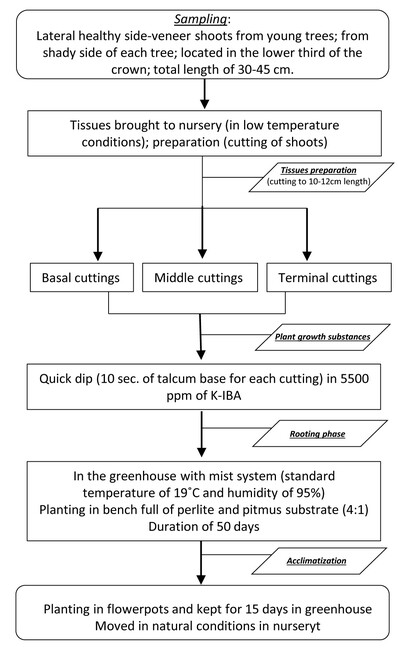
The current experiment addressed the need of simplifying the processes, as well as the minimisation of the propagation time and steps for C. sempervirens f. sempervirens. The experiment was based on the general stages of propagation process, including the following steps where different treatments in each step were tested:
- Culture initiation (tissues preparation): This stage aimed to initiate axenic cultures. It was carried out in the nursery after the field-sampling of tissues. Sampled shoots were divided into three cuttings (basal, middle and terminal part of sampled shoot), where each cutting had a length of approximately 10-12 cm. Remarkable is the fact that in each cut axillary shoot, a bud in its base (footing) was kept up, as well as 4-6 fully formed leaves at the top side of each of the cut shoot part. All shoot cuttings were carried out using a sterilised cutter (with ethanol).
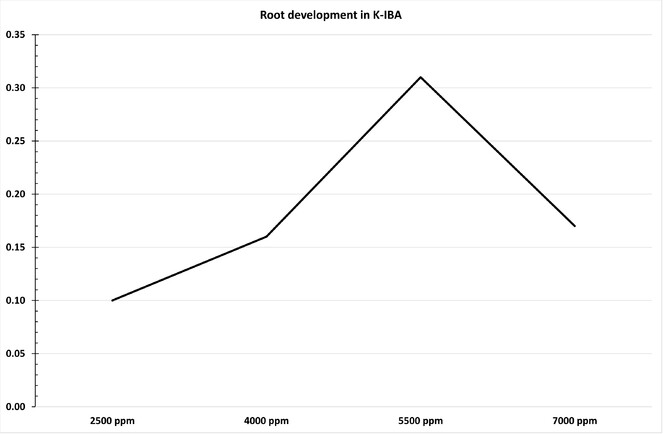
- Plant growth substances: In this stage the masses of shoot tissues were repeatedly subcultured. The “quick dip” method was adopted for this study, due to the type of tissues material. Thus, the talcum base (where bud exists) of cuttings, approximately 4-5 cm, was immersed in a rooting hormones solution for 10 seconds. The indole-3-butyric acid (K-IBA), was used as the rooting hormones solution (K-IBA by Merck KGaA, Darmstadt, Germany). In this experiment four different concentrations of K-IBA were tested (2500 ppm, 4000 ppm, 5500 ppm, 7000 ppm), as well as the neutral concentration without K-IBA (0 ppm; only deionised water) (Hortus USA – see http://www.rooting-hormones.com/IBAmethd.htm).
- Elongation and root induction or development (rooting phase): This stage was designed to induce the establishment of both fully developed roots and plantlets. This stage was carried out in continuation to the previous stage, and, hence, after immersing the basal end of the cut shoot in K-IBA, the shoot was immediately planted for 4-5 cm in root trainers. For this experiment perlite (3-5 mm) with pitmus substrate in a 4:1 ratio was used as the rooting medium, into two different root systems: bench and root trainer. The rooting phase was carried out in a greenhouse with a high-pressure intermittent mist system, at the Athalassa Forest Nursery (Department of Forests; Ministry of Agriculture, Rural Development and Environment, Cyprus). The intermittent mist system assured constant high humidity (95%) in the house, and house temperature of 19ºC on a 24-hour basis (standby). The rooting phase took place for 50 days, and after this period the plantlets were moved to the next stage (see Acclimatization). Notable is the fact that masses of tissues must be repeatedly subcultured under aseptic conditions; therefore shoots were irrigated with a fungicide (i.e. Topam).
- Acclimatization: The last stage of the experiment was the environmental adaptation of plantlets in growing conditions in the field (outside the green house). The shoots that developed root were planted in small plastic flowerpots and kept for 15 days in a greenhouse before being moved to natural conditions in Athalassa Forest Nursery, Department of Forests (Nicosia). Under this framework the young plantlets were cultivated and their viability was monitored for two years (2017 & 2018) in a nursery (Athalassa Forest Nursery).
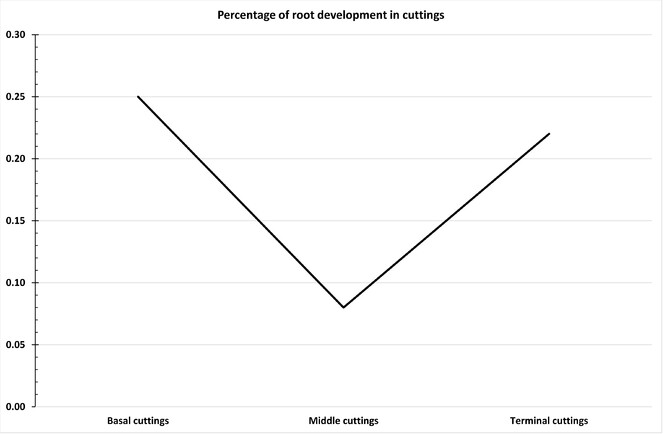
In overview, the current experiment tested the effectiveness of quality propagule production for numerous parameters, namely: (i) germination ability of different parts of the shoot (basal, middle and terminal cuttings), (ii) four different concentrations of K-IBA (2500 ppm, 4000 ppm, 5500 ppm, 7000 ppm; including neutral concentration of K-IBA = 0ppm), (iii) two different rooting systems (bench and root trainer). In total 3000 axillary shoot multiplications were used for this experiment (2400 axillary shoots for test propagation and 600 axillary shoots as neutral markers – Suppl. material
Statistical analyses
For the statistical analyses the rooting shoot development (number of shoots/explant) was recorded after the elongation stage for each tested parameter (treatments) and by first removing from the analysis the samples that were dipped into the solution without K-IBA (neutral samples: 600 axillary shoots). The T-test and analysis of variance (ANOVA) were used for statistical analysis of the results, and in order to check the statistical signal among the different parameters. The statistical analyses were carried out using the IBM SPSS Statistics 20 software.
Data outcomes
The T-test was used for comparing the mean value of successful rooting of the different cuttings of sampled shoots, between the two different rooting systems (bench and root trainer). This test detected statistically significant differentiation between rooting bench (M= 0.20; SD= 0.401) and root trainer (M= 0.17; SD= 0.37), with the mean difference being equal to 0.32 (t= 2.7, df= 2398, F= 18.7, p<0.01). Thus, the tested cuttings had slightly higher (but significant) amount of rooting in bench than in root trainer (Suppl. material
Remarkable is the fact that the survival percentage of new plantlets under the environmental adaptation (acclimatization) in growing conditions outside the green house was: (i) 99% for the first year and (ii) for the second year all (100%) of the survived plantlets of the first year. The acclimatization was carried out in Athalassa Forest Nursery, Cyprus Department of Forests, and followed all necessary maintenance steps for plants in a nursery (watering, manuring, etc.).
Discussion
Today, the expanding of knowledge in the area of developmental tree physiology, in order to improve propagation systems, is important for several purposes in the context of propagation population, clonal forestry, and for conservation purposes, as well as for use οf tree species with specific shapes and growth characteristics in urban forestry (and in agriculture for windbreaks). In all these cases, the purpose of vegetative propagation is to produce clones of elite trees that have been selected from populations of genetically variable siblings (
In the case of C. sempervirens previous studies reported protocols based on micropropagation methods of tissues (especially shoots) (e.g.
A critical point of propagation is root formation, where for this formation, auxins have a central role. Despite the general assumption that different species exhibit varying rooting success for cuttings taken from basal, middle and terminal cuttings (parent stem) (
The protocol and workflow (Fig.
Conclusions
As mentioned above, C. sempervirens is resilent to extreme climate and soil conditions in the Mediterranean region. Thus, the species is highly preferred for several restoration purposes. In all cases, plants macropropagation provides a mass production of plantlets, whilst maximising efficient plantlets multiplication on site, operating in a non-aseptic environment, reducing labour requirements and overcoming a lack of plant stock material at the industrial site (
Acknowledgements
The manuscript is part of the Master’s research of Mr C. Pericleous, referenced as: “Asexual reproduction of Cupressus sempervirens var. pyramidalis in Cyprus with cuttings” (2018), in the School of Pure & Applied Sciences, Open University of Cyprus. The authors thank Prof. I. Vogiatzakis and Dr. D. Sarris, supervisors of Mr. Pericleous for his MSc thesis. Warm gratitude is extended to the Cyprus Department of Forests for assistance with the mist system at Athalassa Forest Nursery. Particularly special thanks are extended to Dr. A. Christou (Chief Conservator of Forests at Department of Forests) and Mr. G. Kyriacou (Forest Officer 1st Grade at Department of Forests). Many thanks to Assoc. Professor P. Mavrikiou (Frederick University) for his input on raw data analyses. Finally, the authors thank the reviewers for their insightful comments on a previous version of the manuscript.
Author contributions
C. Pericleous contributed to the fieldwork and the protocol development. N.-G. H. Eliades carried out the data analyses. Both authors contributed to the manuscript writing.
References
-
Macropropagation and micropropagation of Ziziphus spina-christi.Pesquisa Agropecuária Brasileira40(5):459‑465. https://doi.org/10.1590/s0100-204x2005000500006
-
Is Cupressus sempervirens native in Italy? An answer from genetic and palaeobotanical data.Molecular Ecology18(10):2276‑86. https://doi.org/10.1111/j.1365-294X.2009.04182.x
-
Cupressus sempervirens in Europe: distribution, habitat, usage and threats.In: San-Miguel-Ayanz J, de-Rigo D, Caudullo G, Durrant T, Mauri A (Eds)European atlas of forest tree species.Publication Office of the European Union,Luxembourg,88-89pp.
-
Conifers of the World: The complete reference.Timber Press,London.
-
A handbook of the world’s conifers.Brill,Leiden, Boston. https://doi.org/10.1163/9789047430629
-
An Atlas of the world’s conifers: An analysis of their distribution, biogeography, diversity and conservation status.Brill,Leiden, Boston. https://doi.org/10.1163/9789004211810
-
Induzione in vitro di gemme ascellari della specie Cupressus sempervirens L.Rivista di Ortoflorofrutticoltura Italiana65:293‑299.
-
Micropropagation of two tropical conifers: Pinus oocarpa Schiede and Cupressus lusitanica Miller.In: Henke R, Hugies K, Constantin M, Hollaender M (Eds)Tissue culture in forestry and agriculture.Plenum Press,New York,195-213pp. https://doi.org/10.1007/978-1-4899-0378-5_15
-
Micropropagation of Mediterranean cypress (Cupressus sempervirens L.).In: Jain S, Häggman H (Eds)Protocols for Micropropagation of woody trees and fruits.Springer,Dordrecht, The Netherlands,93-105pp. https://doi.org/10.1007/978-1-4020-6352-7_9
-
Aromatic and spicy plants in Cyprus - From antiquity to the present day.Bank of Cyprus Cultural Foundation,Nicosia, Cyprus. [ISBN978-9963-42-852-6]
-
In pursuit of minimum stress environment for rooting leafy cuttings: comparison of mist and fog.Acta Horticulturae227:211‑216. https://doi.org/10.17660/actahortic.1988.227.34
-
Plant propagation, principles and practices.Prentice Hall,New Jersey.
-
An explant culture of Tassilian cypress Cupressus dupreziana (A. Camus).Forest Ecology and Management8:235‑242. https://doi.org/10.1016/0378-1127(84)90056-2
-
Macropropagation of an endangered medicinal plant, Strychnos henningsii (Gilg), (Loganiaceae) for sustainable conservation.Journal of Medicinal Plant Research2:247‑253.
-
Forest botany.Hellenic Academic Ebook,Athens,619pp. [InGreek]. [ISBN978-960-603-282-0]
-
In vitro plant propagation: A Review.Journal of Forest Science27:61‑72.
-
Protocols for micropropagation of selected economicallyiImportant horticultural plants.Humana Press, Springer Science+Business Media,New York. https://doi.org/10.1007/978-1-62703-074-8
-
Phytosociological and ecological discrimination of Mediterranean cypress (Cupressus sempervirens) communities in Crete (Greece) by means of pollen analysis.Mediterranean Botany40(2):145‑163. https://doi.org/10.5209/mbot.59789
-
The effects of a fogging system on the physiological status and rooting capacity of leafy cuttings of woody species.Trees21(4):491‑496. https://doi.org/10.1007/s00468-006-0121-z
-
The influence of stockplant environment on morphology, physiology and rooting of leafy stem cuttings of Albizia guachapele.New Forests22:213‑227. https://doi.org/10.1023/A:1015668011884
-
Stock plant environment and subsequent adventitious rooting. In: Davis T, Haissig B, Sankhla N (Eds)Adventitious root formation in cutting.Dioscorides Press,Oregon,214-234pp.
-
Genetic variability of the ‘bark canker resistance’ character in several natural provenances of Cupressus sempervirens.Forest Pathology30(2):87‑96. https://doi.org/10.1046/j.1439-0329.2000.00188.x
-
Micropropagation of Cupressus sempervirens L. and Chamaecyparis lawsoniana (A. MURR.) PAR.Silvae Genetica46:87‑96.
-
Autovegetative Gehölzvermehrung. In: Krüssmann G (Ed.)Die Baumschule.Parey Verlag,Berlin,382-449pp.
-
Flora Europaea.I.Cambridge University Press,Cambridge.
-
Micro- and macropropagation of forest trees. In: Geburek T, Turok J (Eds)Conservation and Management of Forest Genetic Resources in Europe.Arbora Publishers,Zvolen,623-650pp.
-
Propagation characteristics of Eucalyptus globulus ssp. globulusstem cuttings in relation to their original position in the parent shoot.Journal of Horticultural Science68(5):715‑724. https://doi.org/10.1080/00221589.1993.11516404
-
Methods and apparatus for the micro-and macropropagation of reed grasses.US Patent 7,052,912 B1,US Patent, US.
-
Micropropagation of quality propagule production in plantation forestry.Indian Journal Biotechnology3:159‑170.
-
Forests of Crete from Antiquity to today.Ministry of Agriculture,Athens.