|
Research Ideas and Outcomes : Grant Proposal
|
|
Corresponding author: Oana Teodora Moldovan (oanamol35@gmail.com)
Received: 28 Nov 2019 | Published: 05 Dec 2019
© 2019 Oana Teodora Moldovan, Rannveig Øvrevik Skoglund, Horia Leonard Banciu, Alexandra Dinu Cucoș, Erika Andrea Levei, Aurel Perșoiu, Stein-Erik Lauritzen
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation: Moldovan OT, Øvrevik Skoglund R, Banciu HL, Dinu Cucoș A, Levei EA, Perșoiu A, Lauritzen S-E (2019) Monitoring and risk assessment for groundwater sources in rural communities of Romania (GROUNDWATERISK). Research Ideas and Outcomes 5: e48898. https://doi.org/10.3897/rio.5.e48898
|
|
Abstract
In the past 100 years, a decreasing rainfall trend has been recorded on Romanian territory, a trend that continues today. Therefore, realistic estimation of the groundwater resources is crucial, especially for the rural communities lacking the economic power to use alternative sources of drinking water. The groundwater sources used by rural communities in Romania generally originate directly from caves, wells or springs with no proper evaluation of the water quality. Groundwater is exposed to different pollutants, as bats' guano in caves, fertilizers in agricultural areas or livestock (cattle, sheep, goats, etc.) farms on the surface. On the other hand, the water extracted directly from inside the caves is affecting groundwater ecosystems, highly vulnerable to any human impact and neglected by European legislation so far. The project aims to monitor, during two consecutive years, groundwater sources with different degrees of above- and underground pollution, from different regions of Romania. To achieve the goals of the project, a multidisciplinary monitoring strategy that will include measurements of hydrological, physico-chemical and biological (microbiology and aquatic invertebrates’ assessment) parameters alongside the quantification of radon and stable isotopes, rainfall or possible inflows of water. The specific outcomes of this project are: i) to test, develop and validate a new, more rapid and efficient method for monitoring and risk assessment of groundwater sources – and not only – by using molecular techniques, and propose this method to the water agencies in Romania; ii) to propose for Romanian authorities to implement a harmonized coherent methodology to measure radon concentration in water, as a consequence of EURATOM Directive; and iii) to educate local communities that are using groundwater as source for drinking water and raise young people’s awareness on the benefits of ecosystem services provided by the groundwater.
Keywords
groundwater, springs, microbiology, chemistry, stable isotopes, radon, risk assessment, ecosystem services, rural communities, Romania, Norway
Overall aim of the project
The research aims of the GROUNDWATERISK project (Fig.
The proposed OBJECTIVES are:
- Interdisciplinary evaluation of groundwater ecosystems used as sources of drinking water in rural communities
- Finding the most feasible method for groundwater microbiological monitoring to ensure water quality for human use and to better protect against possible outbreaks of pathogenic bacteria caused by contaminated drinking water
- Risk assessment for the groundwater sources used by rural communities
- Assessment of the groundwater ecosystem services to enhance conservation practices in rural communities.
The target group of our research are the rural communities that are not connected to a public water supply. According to
Key targets to be achieved in the project are related to the proposed objectives:
- An evaluation of the quality and sustainability of groundwater sources used by the rural communities across Romania;
- Establishing the most feasible method for microbiological monitoring and radon concentration measurements of drinking water to be applied by responsible agencies;
- To assess the environmental and human-induced risks for groundwater sources in different regions of Romania;
- To educate people for the protection of their local source of water and its sustainable use.
State of the art
Groundwater is defined in the Water Framework Directive (WFD), Directive 2000/20/EC, as “all water which is below the surface of the ground in the saturation zone and in direct contact with the ground or subsoil”. Groundwater is the largest supply of water for human consumption with 97% of all freshwater on the Globe being underground (
Groundwater harbors a unique and vulnerable ecosystem characterized by lack of light and primary producers, relatively stable physico-chemical conditions and poor nutrients content - unless human-induces changes are interfering. The poor food resources originate from the surface as particulate organic carbon (POC) or dissolved organic carbon (DOC) and microbial activity, which is low per volume of water. Groundwater animals in Romania are invertebrates, mostly Crustaceans, which have particular adaptations to life underground: lack or reduction of eyes, depigmentation, elongated appendages, fine body shape, slow metabolism and high vulnerability to high variation of their physico-chemical environment. They are used in ecological studies as an biondicators of water quality, their presence and diversity indicating the ecological state of the environment.
Waterborne diseases are a global burden which is estimated to cause more than 2.2 million deaths/year and an even higher number of recorded and unrecorded illnesses (
For the monitoring of the quality of water used for drinking, irrigation and bathing along the physico-chemical parameters, the examination of the microbiological standard parameters is mandatory: EU-Surface & Drinking Water Directive 75/440/EEC and EU-Bathing Water Directive 76/160/EEC. Nevertheless, the methods applied in the microbiological monitoring of waters are outdated and underestimate the level of microbial pathogens (e.g. the multiple-tubes method is used as a standard for Romanian and other European countries water estimation of pathogen bacteria such as E. coli) while groundwater microbiological monitoring is not performed at all. Although molecular techniques might improve the identification and abundance estimation of these pathogens, several disadvantages such as the lack of standardization of protocols and sample processing are still a challenge (
Radon (222Rn), found in soil, rocks and water all over the Earth, is listed by the World Health Organization as the second leading cause of lung cancer after cigarette smoking. Areal variations of radon levels in houses depend on numerous factors, such as geological features, environmental parameters or occupational patterns. Most of the cancer risks from radon in drinking water arise from the transfer of radon into indoor air, and the exposure through inhalation (
Stable isotopes used for environmental studies. Due to the direct relationship between air temperature and δ18O and δ2H in rainfall and spring water, we can establish the moment when karst aquifers recharge occurs and the delay between the moment of surface rainfall and runoff and underground recharge (e.g., by determining the time difference between the moment of winter precipitation with very low δ18O and δ2H and the moment these low values are registered in the underground streams). The hypothesis is that, for hydrokarstic systems with diffuse feeding, there is a several months interval between rainfall and the moment when water reaches the subsurface karst. For karst systems fed directly through ponors and caves, the rainfall (including the potential contaminants) reaches the subsurface within days.
Metabarcoding of water samples and detection of pathogens. By next-generation sequencing methods, high diversity of prokaryotes pertaining to both Archaea and Bacteria has been detected in groundwater, including members of Crenarchaeota, Euryarchaeota, Proteobacteria, Planctomycetes, Actinobacteria, Chloroflexi, Chlorobi, Bacteroidetes, Firmicutes and Cyanobacteria phyla (
In areas with high population density and/or intensive land use, groundwater is vulnerable to contamination, as various pathogenic microorganisms may enter groundwater due to septic systems, livestock manure, contaminated wells or recharge waters, etc. Groundwater contaminants detected through DNA-based studies include members of Xanthomonadales (known crop pathogens), Pseudomonadales (components of biofertilizers), and Burkholderiales (Comamonadaceae) used as biocontrol agents in agriculture (
Risk analysis requires a holistic approach to assess stress and vulnerability of groundwater resources. Risk assessment of water supplies aims at:
- identifying causes that may threaten the quality of the water supply;
- assess whether the harm can be eliminated; and if not
- suggest preventive or protective measures that can control and reduce the risk.
In the case of lacking (economic) resources, identification of highest risks is essential so that these risks can be handled first.
The management of groundwater contamination is a very difficult task due to the spatial heterogeneity of the aquifers and the natural processes in the soil and the unsaturated and saturated zones of the karst (
Groundwater in karst terrain is vulnerable to contamination due to the concentrated channel flow with low transit time and little self-purification within the karstic system. The European Approach to karst vulnerability and risk mapping of karst aquifers, by the COST Action 620, define two central terms: the intrinsic vulnerability of groundwater to contaminant which considers the geological, hydrological and hydrogeological characteristics of the karst area, and the specific vulnerability that accounts for the properties of the contaminant or group of contaminants (
Karst aquifers are unique in the way that enlarged fissures, conduits and caves provide habitats for macro and microorganisms and may give humans direct access to the water resource inside the aquifer. Biological contamination inside the karstic system may be an important threat to the water quality and safety. An evaluation of the degree of karstification and the flow system development as well as human and biological activity in accessible caves is a second approach in the risk assessment. Risk and vulnerability maps are useful tools for limited monitoring resources and in such areas a major effort is required to avoid or mitigate the impact of human activities on the environment (
Development of a microbial contamination susceptibility model for private groundwater sources has been carried out by assessing the presence of thermotolerant coliform (TTC) in groundwater (
Ecosystem services. Ecosystem services are vital to human survival and wellbeing, and the judicious management of these systems being essential. Ecosystem service indicators are increasingly recognized as a key part of assessing whether ecosystem services are being managed appropriately and used sustainably (
The important outcomes of the project
Project contribution beyond the state-of-the-art
The research we propose has several components that are new to science and others which were never applied in Romania, as follows:
- first overview of the quality of groundwater sources used as drinking water in Romania by using multidisciplinary indicators (physical, chemical, biological and microbiological);
- identifying a filter for microbiological sampling that will be effective not only in detecting the presence/absence of microbial pathogens but also in identifying the presence of pathogens at low concentrations;
- new protocol for groundwater microbiological monitoring and accurate identification based on molecular methods;
- first radon analysis on water and first derived map on radon risks in Romania;
- identification of groundwater invertebrates that can be used as indicators of water quality;
- identification of microorganisms possibly involved in water purification;
- first hydrological studies at a country level that will use stable isotopes as indicators of water origin and water residence time underground;
- first risk maps for groundwater sources in Romania and the first maps that will include microbial pathogens and radon in the analysis;
- first maps of groundwater ecosystem services in Romania.
Technical milestones and expected results
The milestones and the expected results are as follows:
- Compiling a database with all the results (physico-chemical, radon, stable isotopes, biological, microbiological) after the monitoring period. This database will also contain the identified invertebrate species, inferred pathogenic and nonpathogenic microorganisms in each of the studied sites and will provide the basis for the risk analysis and the ecosystem services approaches;
- Choosing both the optimal filter and filtration methods for retaining of pathogen's biomass and the optimal test for pathogens in groundwater in terms of costs, accuracy, efficiency and flexibility. The filter and the test will be used to validate a protocol for best practices in microbiological monitoring for groundwater sources of drinking water. The protocol will be published and distributed to water agencies;
- Risk analysis for all the studied sites. It will highlight the vulnerabilities of local groundwater ecosystems and the possible risks for the human populations;
- A GIS model with ecosystem services in Romania. A simplified model will be distributed in brochures and leaflets addressed to rural communities and local schools and for the training of the personnel of water and health agencies;
- Conferences held in local communities and schools on the need to protect groundwater sources and the importance of groundwater ecosystems;
- A guideline on measurement procedures for the determination of radon concentration in water;
- Publication of results in high impact factor journals and presentation of results at national and international conferences;
- Implication of students and young researchers in new fields of research and in interdisciplinary activities.
Methodologies to be used
A. Sites selection. Sites will be selected in different regions of Romania, with different surface use, different origin of the water (surface – short flow underground, surface – long flow underground, aquifer), various hydrology, various human impacts on the surface, etc. Samples will be taken seasonally during a 2-years period.
B. Sampling. Water will be collected in special bottles for chemical analysis, microbiology, stable isotopes and radon:
- Physico-chemical measurements. Electrical conductivity, temperature, pH, oxygen and flow speed will be measured in situ. For the other chemical parameters 0.5-1 L water sample will be collected in PTFE bottles. Samples will be stored and conserved using standardized protocols (metals by acidulating with concentrated nitric acid, organics by refrigeration).
- 25 ml aliquot of water will be sampled for stable isotope analyses. Every three months, samples will be collected from the stations and transported to the laboratory for analyses.
- Aquatic invertebrates will be sampled by using a 60 µm planktonic net directly from springs for a standardized quantity of water. All samples will be preserved in 95% ethanol.
- Microorganisms will be sampled on commercial films with growth media. At each sampling site, 1 mL of water will be directly applied, with a sterile plastic pipette, onto the growth medium surface, and then transported, on ice, to the laboratory for incubation.
C. Identification and molecular analysis of invertebrates. Samples will be sorted under the optical microscope and identified at least at fauna group level, except for the crustaceans that will be identified at species level. Specimens will be also sent to specialists for identification. Amphipods will be analyzed by molecular methods and a phylogeny with all the obtained sequences will be build-up.
D. Molecular identification of microorganisms and profiling of water-borne pathogens. We will attempt to explore the molecular diversity of putative pathogens in groundwater by sequentially and complementary using three different molecular biology approaches:
(a) Commercial films. The plates will be transported at constant temperature in a cooler bag, and placed in incubators. The plates will be analyzed at 24-hour intervals, the results being expressed in the total readings after five days (
(b) Metabarcoding. To rapidly screen for the putative diversity and abundances of bacteria (and Archaea altogether), the amplicon sequencing (or metabarcoding) technique targeting the highly conserved, taxonomic relevant 16S rRNA gene will be employed. Raw sequence data obtained by this approach will be analyzed. Several processing steps of joining pair-end reads, quality filtration, dereplication, singleton and chimera removal will provide good quality sequences for taxonomic assignment. Recently released DNA sequence databases are available and can be used for establishing taxonomic diversity (Silva 132, Greengenes ’13-8’, Ribosomal Database Project). The metabarcoding approach allows overpassing the limitations of culture-dependent techniques, being a cost-effective and fast assessment, providing data on the entire prokaryotic community including ‘unculturable’ or fastidious microbes. We expect that the metabarcoding approach will accurately resolve the microbial community composition down to family and genus level. Thus, the presence and abundances of bacterial families comprising pathogenic members (e.g., the Gram-negative Enterobaceriaceae, Campylobacteriaceae, Aeromonadaceae, the Gram-positive Streptococcaceae, Staphylococcaceae, etc.) will be quickly evidenced.
(c) Quantitative PCR (qPCR). If possible, presence of pathogens is inferred by method (a), the more sensitive qPCR assay will be performed targeting selected marker genes (
All molecular methods described above will be applied on the same samples collected from the same sites following the sub-splitting of membrane filters. The environmental DNA will be extracted from biomass retained on hydrophilic filter membranes with 0.22 µm pore size and large diameter (90 mm) under negative pressure (i.e., generated by vacuum pump). The filtered groundwater volumes (up to 15 L expected) will depend on how quick the filter membranes will be clogged. Each membrane will be then separated into slices needed for DNA extraction for further molecular analysis. The unused extracted DNA will be stored under freezing conditions.
E. Radon measurements. The radon measurements in water will use the Luk-VR system, which involves connecting a VR-scrubber to a radon detector. This method requires mixing of the dissolved radon from the water sample with the air above the water in the volume of the glass vessel. Following this procedure, the sample of air is transferred to the Luk 3P, and measured by the Lucas cell method.
The water samples will be collected in glass bottle of 0.5 L, fully filled and tightly sealed and transported to the laboratory for measurement purposes. The time interval between sampling and measurement is recommended to be of maximum 48 hours, in which case the half time must be considered and corrections are made accordingly
F. Stable isotopes. Precipitation will be collected continuously using specially designed collectors, constructed according to IAEA specification. A 3-L HDPE plastic canister is fitted with a funnel, prolonged with a plastic tube, channeling water to the bottom of the container. Excess air escapes the canister through to a narrow, 3 m long plastic tube, to minimize air exchange between the container and the outside environment. The funnel will be “sealed” with table tennis balls, to restrain insect access, but allow water collection. At the end of each month, the amount of water in the canister will be measured (to be compared with data provided by the Romanian National Meteorological Administration), and a 25 mL aliquot of water will be sampled for stable isotope analyses. The aliquot will be stored in HDPE scintillation vials at 4oC. In winter, snow samples will be collected after each event, allowed to melt at room temperature in closed vessels, and stored in similar manner to liquid samples. River water will be collected at the end of each month, from boats or bridges, from ca. 15 cm below water table, in 25 mL HDPE vials and refrigerated until analysis. Groundwater will be sampled from dug wells and deep wells (where available), as well as from springs.
Climate and hydrologic data will be provided by the respective national authorities. Where such data will not be available, we will install temperature data loggers, measuring air temperature with hourly resolution. An ongoing study has shown that there are no systematic differences between data from the national meteorological service and data provided by the loggers.
Stable isotope analyses will be performed in the laboratory. Prior to analysis, samples are filtered using 0.45 nm nylon microfilters. The results are calibrated against two internal standards (Greenland and Hawaii waters) and checked against a third one (Romanian water). Per laboratory internal regulations, an aliquot from each sample will be stored in 3 mL paraffin-sealed, screw-cap, glass vials.
G. Chemical analysis. Chemical parameters will be determined by standardized or alternative methods. Trace metals (Cd, Cu, Cr, Pb, Ni) will be determined by ICP-MS, mercury by atomic fluorescence spectrometry, major cations and P by ICP-OES and anions (nitrates, nitrites, phosphate, sulphates) by ion chromatography. Organic carbon ant total nitrogen will be determined by a combustion analyzer with NDIR and respectively chemiluminescence detector. Chemical oxygen demand (COD) and alkalinity will be determined by volumetric, total dissolved solids by gravimetric, while phenol index, ammonium and cyanides by spectrophotometric methods. Whenever applicable, standardized methods will be applied. For organics, water will be sampled in glass bottles, while for metals and anions in polyethylene bottles. Samples will be stored and conserved using standardized protocols (metals by acidulating with concentrated nitric acid, phenols by addition of phosphoric acid, organics by refrigeration). Where recommended, analysis (COD, DOC, alkalinity) will be carried out in the day of sampling. Quality control will be made by reference materials analysis and inter-laboratory trials.
H. Risk analysis. We will use an integrated method for assessing groundwater contamination risk, based on the interaction between natural conditions and human activities, and by using analytical and numerical tools within a GIS framework. Different factors along the contaminant pathway from source to groundwater will be incorporated in the GIS database and analytical and numerical tools in GIS software will be used. The spatial groundwater contamination will be classified into categories based on the degree of risk (very high, high, moderate, low and very low). This classification is performed by considering the factors that influence groundwater contamination and assigning relative weights to them. This process is performed in a GIS environment in which thematic maps are produced for every factor. The linear combination of the thematic maps and the selection of the weights yield the final map of groundwater contamination risk.
In addition to the 30 Romanian sites, the project will also benefit from monitoring and investigation of two sites in Northern Norway where karst springs serve as water supplies for small communities. These two sites will be used during the project as models for the Romanian sites and as school-sites for the Romanian students. At both sites, cave systems upstream have been surveyed and investigated.
I. Ecosystem services’ evaluation. Identifying indicators takes a combination of scientific rigor and creative thinking. Creative thinking may be a surprising skill in this context, but the indicators with the greatest impact are often produced by combining different kinds of data. Scientific rigor is necessary to identify indicators that are conceptually valid and defensible for their purpose. In our case, the microbiological aspect will be important, because not only pathogens will be identified, but also microorganisms that could be of importance for water purification.
Data relevant for developing ecosystem service indicators will be available from our database. A wide range of models that exist for monitoring ecosystem services will be tested, as for example:
Co$ting Nature that calculates the spatial distribution of ecosystem services for water, carbon, hazard mitigation and tourism and combines these with maps of conservation priority, threatened biodiversity and endemism to understand the spatial distribution of critical ecosystems (
J. Communication of the obtained results to local communities and water and health agencies is an important component of our project. The obtained results will be published and also presented to the general public on the project site. The editing of a brochure and a leaflet for the local communities and the children in the respective communities will be presented in a friendly manner. We will present not only the obtained results but also impact messages regarding the conservation of groundwater sources. Public conferences and training of people from water and health agencies are also part of our strategy for the improvement of groundwater monitoring and protection.
During our field work we will also try to establish contacts in the local communities and involve the children and young people in our monitoring activities.
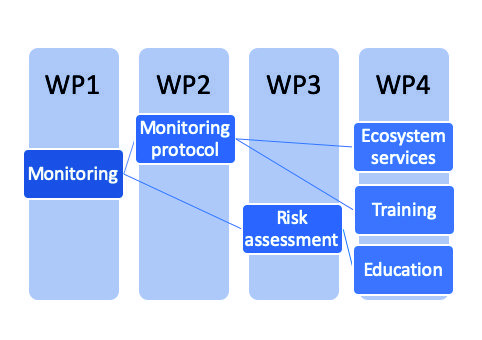
Project structure
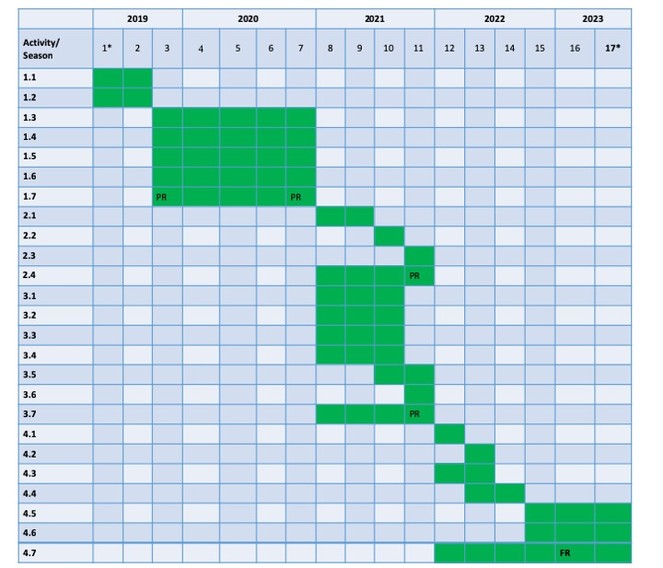
The project is structured in 4 Work Packages (WPs) distributed along the 48 months of the project (June 2019 – May 2023). The project is split in seasons as we will do the sampling seasonally and all the work will be organized, at least for the first 2 years, according to the sampling campaigns (see also Fig.
WP 1. Evaluating the groundwater ecosystems used as sources of drinking water
Objectives. Monitoring in different sites across Romania and Norway to ensure a multidisciplinary view on the groundwater quality used by rural communities as drinking water (wells, caves and springs).
Activities:
- Selection of sites to be studied and preliminary sampling;
- Testing of an efficient water filtering method for pathogens;
- Continuous sampling of precipitation for isotopic analysis;
- Seasonal sampling of microorganisms and invertebrate fauna in water for two successive years and on-site measurements;
- Laboratory analysis;
- Molecular identification of microorganisms and fauna identification in the laboratory by use of conventional morphological methods;
- Project management.
Deliverables. Press conference, Stations established, Kick-off meeting, Best filter for water pathogens identified, Common field-work in Romania, Devices installed, Common field-work in Norway, Project site opened for the public access, First data introduced in the database, Workshop on methods in groundwater monitoring, Database completed, Results dissemination, Phase report.
WP 2. Improving the methods for groundwater microbiological monitoring to better protect against possible outbreaks of pathogen bacteria in the drinking water
Objectives. Testing and validating a method for groundwater microbiological monitoring for water sources.
Activities:
- Laboratory testing of different methods for monitoring pathogenic bacteria;
- Comparing the results of the three methods from all points of view;
- Validating monitoring protocols on groundwaters of different qualities;
- Project management.
Deliverables. Protocol for microbiological monitoring, Patent documentation, Results dissemination, Phase report.
WP 3. Risk assessment for the improvised groundwater sources used by rural communities
Objectives. Survey at the surface of areas and water basins where the monitored sites are located and production of GIS maps where the risks for each of the studied site will be highlighted.
Activities:
- Assessment of the quality and presence of a cover layer;
- Identification of swallow holes and inlets on the surface;
- Assessment of the human and agricultural activities on the surface;
- Evaluation of the radiological risk for groundwater;
- Production of GIS-based vulnerability maps;
- Risk reports for the study cases;
- Project management.
Deliverables. Common field-trip in Romania, GIS vulnerability maps, Risk assessment reports, Report on radon, Results dissemination, Phase report.
WP 4. Assessment of the groundwater services in romania for enhancing environmental awareness and education
Objectives. Raise the interest of the rural communities for the ecosystem services provided by groundwater, including the important drinking water source service, and develop indicators and maps for these services by using the obtained results.
Activities:
- Developing groundwater ecosystem services indicators;
- Mapping the ecosystem services in the studied areas;
- Developing a tool for good practices on measurement procedures for the determination of radon concentration in water;
- Printing brochures and leaflets for local communities (for adults and for children);
- Training for responsible water and health agencies for the best monitoring method and protection of groundwater sources;
- Educating the local communities on the potential health impact associated with the pollution/radon from groundwater sources;
- Project management.
Deliverables. Ecosystem services indicators, Inter-comparison report for radon, GIS model of ecosystem services, Tool for good practices for radon, End of project workshop, Training for the representatives of the water and health Romanian agencies, Leaflets/brochures edited and distributed in local communities, Conferences for local communities, Results dissemination, Final report.
Acknowledgements
This proposal was reviewed by a team of international reviewers as a submitted research proposal before being awarded funding.
Funding program
The EEA Grants CALL FOR PROPOSALS 2018 – Collaborative Research Projects
Grant title
Monitoring and risk assessment for groundwater sources in rural communities of Romania (GROUNDWATERISK)
Hosting institution
The Romanian Academy - Cluj Branch, Cluj Department of the Emil Racovitza Institute of Speleology, Romania
University of Bergen, Norway
Babeș-Bolyai University of Cluj, Romania
National Institute of Research and Development for Optoelectronics INOE 2000, Research Institute for Analytical Instrumentation Subsidiary, Cluj-Napoca, Romania
Author contributions
OTM wrote the proposal, ROS contributed to the risk assessment part, HLB contributed to the molecular biology methods, ADC contributed to the radon part, EAL contributed to the chemical methods, AP contributed to the stable izotopes method, SEL contributed to the Norwegian selection of sites. All authors corrected and approved the final manuscript.
Conflicts of interest
There is no conflict of interests.
References
-
Assessment of intrinsic vulnerability to contamination for Gaza coastal aquifer, Palestine.Journal of Environmental Management88:577‑593. https://doi.org/10.1016/j.jenvman.2007.01.022
-
Novel approach to microbiological air monitoring in show caves.Aerobiologia34:445‑468. https://doi.org/10.1007/s10453-018-9523-9
-
Microbiology of drinking water production and distribution.1st.John Wiley & Sons,NJ, USA.
-
Measuring ecosystem services: guidance on developing ecosystem indicators.Report UN Environment
-
The combined approach when assessing and mapping groundwater vulnerability to contamination.Journal of Water Resource and Protection2:14‑28. https://doi.org/10.4236/jwarp.2010.21003
-
Risk assessment of radon in drinking water.National Research Council,296pp.
-
Radon in water from Transylvania (Romania).Radiation Measurements43:1423‑1428. https://doi.org/10.1016/j.radmeas.2008.05.001
-
Council Directive 2013/51/Euratom of 22 October 2013 laying down requirements for the protection of the health of the general public with regard to radioactive substances in water intended for human consumption.EC
-
Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption.EC
-
Treaty consolidated version of the Treaty establishing the European Atomic Energy Community (2010/C 84/01) Articles 35-35.EC
-
EU Commission recommendation of 20th December 2001 on the protection of the public against exposure to radon in drinking water.Official Journal of the European Commission.
- https://appsso.eurostat.ec.europa.eu/nui/show.do?dataset=env_wat_pop&lang=en. Accessed on: 2019-10-31.
-
Groundwater ecology.Academic Press,San Diego. https://doi.org/10.1016/B978-0-08-050762-0.50008-5
-
The PI method–a GIS-based approach to mapping groundwater vulnerability with special consideration of karst aquifers.Zeitschrift für angewandte Geologie46:157‑166.
-
Survival of pathogenic and indicator organisms in groundwater and landfill leachate through coupling bacterial enumeration with tracer tests.Desalination4:162‑168. https://doi.org/10.1016/j.desal.2010.05.007
-
The links between biodiversity, ecosystem services and human well-being.In: Raffaelli D, Frid C (Eds)Ecosystem ecology: A new synthesis.Cambridge University Press
-
Development of a microbial contamination susceptibility model for private domestic groundwater sources.Water Resources Research48:W12504.
-
Doses to organs and tissues from radon and its decay products.Journal of Radiological Protection22:389. https://doi.org/10.1088/0952-4746/22/4/304
-
Wells provide a distorted view of life in the aquifer: implications for sampling, monitoring and assessment of groundwater ecosystems.Scientific Reports7:40702. https://doi.org/10.1038/srep40702
-
Environmental risk mapping of pollutants: state of the art and communication aspects.Science of the Total Environment408:3899‑3907. https://doi.org/10.1016/j.scitotenv.2009.10.045
-
Intrinsic vulnerability, hazard and risk mapping for karst aquifers: a case study.Journal of Hydrology364:298‑310. https://doi.org/10.1016/j.jhydrol.2008.11.008
-
Capturing and quantifying the flow of ecosystem services.In: Silvestri S, Kershaw F (Eds)Framing the flow: Innovative approaches to understand, protect and value Ecosystem Services across linked habitats.UNEP World Conservation Monitoring Centre,Cambridge, UK.
-
Trading off agriculture with nature's other benefits, spatially.In: Zolin CA, Rodrigues RdA (Eds)Impact of Climate Change on Water Resources in Agriculture.CRC Presshttps://doi.org/10.1201/b18652-10
-
Legea nr. 301/2015 privind stabilirea cerințelor de protecție a sănătății populației în ceea ce privește substanțele radioactive din apa potabilă.Parlamentul României
-
Microbial contamination of groundwater at small community water supplies in Finland.Ambio40:377‑390. https://doi.org/10.1007/s13280-010-0102-8
-
Ecosystem services: Exploring a geographical perspective.Progress in Physical Geography35:575‑594. https://doi.org/10.1177/0309133311423172
-
Waterborne pathogens: Detection methods and challenges.Pathogens4:307‑334. https://doi.org/10.3390/pathogens4020307
-
Proposed methodology of vulnerability and contamination risk mapping for the protection of karst aquifers in Slovenia.Acta Carsologica36:397‑411.
-
Assessing ecosystem service change & its impacts on human wellbeing: A national pilot of indicator approaches and data. Report No. CSIR/NRE/ECOS/IR/2014/0016/B.Council for Scientific and Industrial Research
-
Molecular detection of waterborne microorganisms.In: Staff A (Ed.)Waterborne Pathogens.2nd.American Water Works Association
-
A conceptual framework to assess the effects of environmental change on ecosystem services.Biodiversity and Conservation19:2823‑2842. https://doi.org/10.1007/s10531-010-9838-5
-
LPJmL4 – a dynamic global vegetation model with managed land – Part 2: Model evaluation.Geoscientific Model Development11:1377‑1403. https://doi.org/10.5194/gmd-11-1377-2018
-
LPJmL4 – a dynamic global vegetation model with managed land – Part 1: Model description.Geoscientific Model Development11:1343‑1375. https://doi.org/10.5194/gmd-11-1343-2018
-
Functional diversity of microbial communities in pristine aquifers inferred by PLFA- and sequencing-based approaches.Biogeosciences14:2697‑2714. https://doi.org/10.5194/bg-14-2697-2017
-
Metagenomic comparison of microbial communities inhabiting confined and unconfined aquifer ecosystems.Environmental Microbiology14:240‑253. https://doi.org/10.1111/j.1462-2920.2011.02614.x
-
Groundwater Risk Assessment Model (GRAM): Groundwater Risk Assessment Model for wellfield protection.Water5:1419‑1439. https://doi.org/10.3390/w5031419
-
Investigating antibiotics, antibiotic resistance genes, and microbial contaminants in groundwater in relation to the proximity of urban areas.Environmental Pollution236:734‑744. https://doi.org/10.1016/j.envpol.2018.01.107
-
Use of geographic information systems for assessing groundwater pollution potential by pesticides in Central Thailand.Environment International29:87‑93. https://doi.org/10.1016/S0160-4120(02)00149-6
-
Proposed method for groundwater vulnerability mapping in carbonate (karstic) aquifers: the COP method: application in two pilot sites in Southern Spain.Hydrogeology Journal14:912‑925. https://doi.org/10.1007/s10040-006-0023-6
-
A methodology for adaptable and robust ecosystem services assessment.PLOS One9:e91001. https://doi.org/10.1371/journal.pone.0091001
-
Guidance for drinking water quality. Recommendations.3rd.World Health Organization,Geneva.
-
Handbook on indoor radon, a public health perspective.World Health Organization,Geneva.
-
World health statistics 2015.World Health Organization,Geneva.
-
Vulnerability and risk mapping for the protection of carbonate (karst) aquifers. COST Action 620.Office for Official Publications of the European Communities,Luxembourg.