|
Research Ideas and Outcomes : Research Article
|
|
Corresponding author: Sulav Paudel (sulavpaudel111@gmail.com)
Received: 03 May 2019 | Published: 03 Jun 2019
© 2019 Sulav Paudel, Istvan Miko, Edwin Rajotte, Gary Felton
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation: Paudel S, Miko I, Rajotte EG, Felton GW (2019) Lyonet’s gland of the tomato fruitworm, Helicoverpa zea (Lepidoptera: Noctuidae). Research Ideas and Outcomes 5: e35906. https://doi.org/10.3897/rio.5.e35906
|
|
Abstract
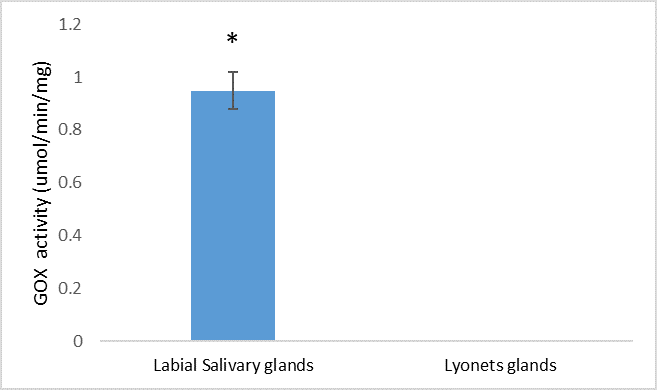
The Lyonet’s gland is a widespread accessory labial gland in Lepidoptera. Although its function is ambiguous, the Lyonet’s gland arguably plays an important role in silk production. Our knowledge of the Lyonet’s gland in heliothine species is extremely limited; it is reportedly missing from Helicoverpa armigera and Heliothis virescens, whereas it is reportedly reduced in size in Helicoverpa zea. Using confocal microscopy and brightfield imaging, we show that the Lyonet’s gland in Helicoverpa zea is present and the size is relatively enlarged relative to other lepidopterans. We also examined whether glucose oxidase, an abundant enzyme found in labial salivary gland is also present in the extracts of Lyonet’s gland, but we found no evidence of that. Based on the size and accessibility of the Lyonet’s gland, future studies should include transcriptomic and proteomics studies in H. zea to provide evidence for potential functions.
Keywords
Lyonet's gland, tomato fruitworm, confocal microscopy and brightfield imaging, glucose oxidase
Introduction
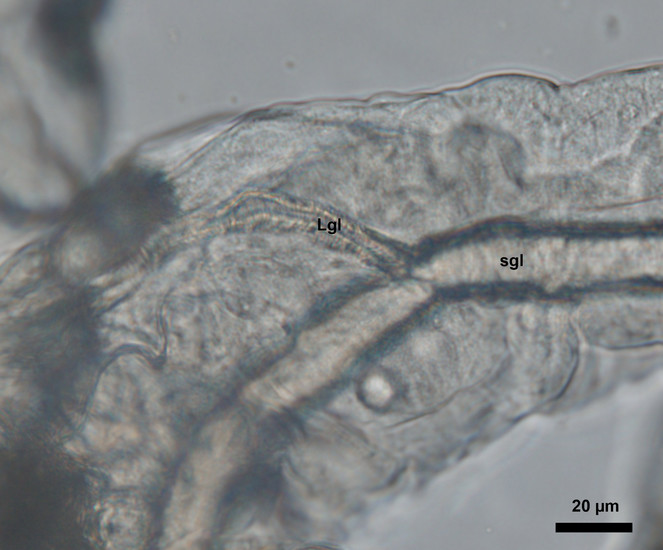
The Lyonet’s gland is an accessory gland located at the proximal region of the silk or labial gland (
Many heliothine moths (Lepidoptera: Noctuidae) are major insect pests in several crops worldwide, and disruption of their silk production could have potential as a management strategy. While there are several studies on the main silk glands (
The aim of the present study was to investigate the presence of the Lyonet’s gland in H. zea using dissection, bright field and confocal laser scanning microscopy. If present, we will also examine whether active glucose oxidase, a highly abundant enzyme found in labial salivary gland of H. zea (
Material and methods
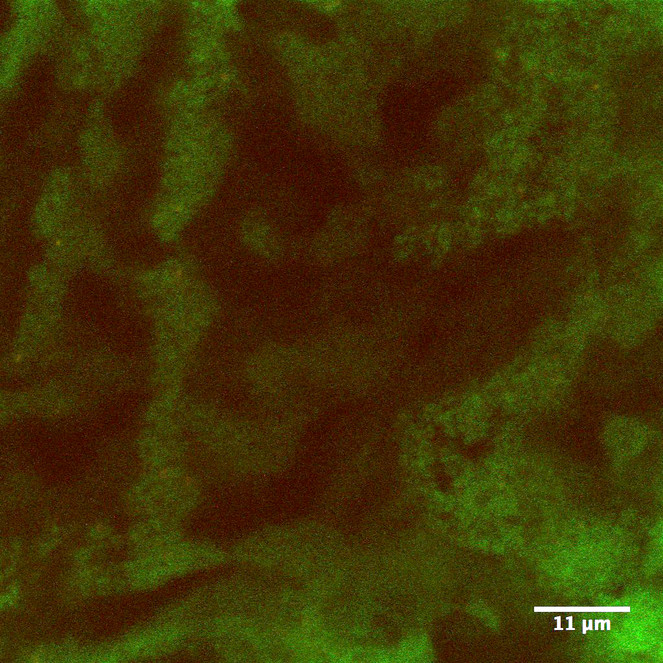
Fifth instar Helicoverpa zea were dissected in 0.1 M phosphate buffer (pH 7.4). Lyonet’s glands were fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer, and 5% sucrose for 24 hours on room temperature, washed in phosphate buffer, transferred and imaged in glycerol on concavity microscope slides. The glands were imaged with an Olympus BX41 compound microscope equipped with a Cannon EOS 70D SLR digital camera and with an Olympus FV10i Confocal Laser Microscope using two excitation wavelengths: 473 nm, and 559 nm. Auto-fluorescence was detected using three channels with emission ranges of 490–590 nm (green), and 570–670 nm (red), respectively. Volume rendered micrographs and media files were generated with ImageJ (
Glucose oxidase (GOX) activity in Lyonet's gland was assayed using six pairs of Lyonet's gland and labial salivary glands (+ve control) collected from 5th instar caterpillars. Glands were homogenized with phosphate buffer (0.1 M, pH 7.0), and the supernatant was then collected after centrifugation (4 °C, 7,500× g, 10 min) to quantify GOX enzyme activity following
Results
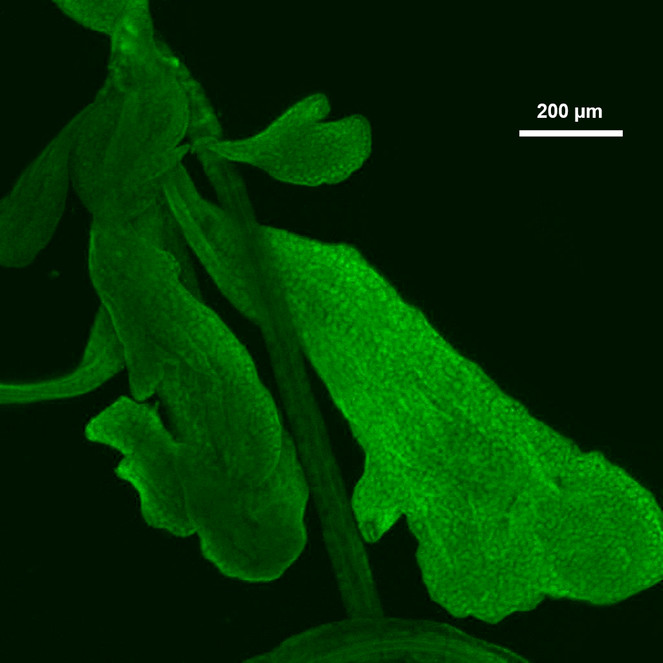
Based on our observation, the Lyonet’s glands in Helicoverpa zea larvae were branched from the proximal region of the silk glands (Figs
Discussion
The structure of the Lyonet's gland in H. zea was different from what was illustrated by
The relative size of this gland in H. zea, which is 2–3 times larger than the same gland in Bombyx mori suggests that the gland plays an important role in the biology of H. zea. The labial glands have multiple function in Llepidoptera; in addition to silk formation saliva produced by the glands may be involved with digestion, detoxification, lubrication of the mouthparts, suppression of plant defenses, etc. (
Acknowledgements
The current work is the result of the” Know Your Insect” 2017 fall graduate course of the Entomology Department at the Pennsylvania State University. We thank Adam Rork, Asifa Hameed, Po-An Lin, Ching-Wen Tan, and Maria Perezsandi for thoughtful discussions, literature search and assistance with dissections. SP is supported by the United States Agency for International Develpoment (USAID) Integrated Pest Management Innovation Lab (IPM IL) program.
Author contributions
SP and IM: Conceptualization, investigation, and writing
EGR and GWF: Supervision, review and editing
Conflicts of interest
There is no conflict of interest.
References
-
The ultrastructure and functions of the silk gland cells of Bombyx mori.Insect Ultrastructure323‑364. https://doi.org/10.1007/978-1-4613-2715-8_9
-
Ultrastructures of silk glands and cocoon filaments from the Mexican silkmoth, Eucheira socialis.International Journal of Wild Silkmoth & Silk8:65‑72.
-
Étude morphologique de l’appareil séricigene des larves de quelques espèces de Trichoptères et conséquences physiologiques immédiates.Bulletin de la Société Zoologique de France88:556‑558.
-
Comparative morphology and histology of the larval digestive system of two genera of Noctuidae (Lepidoptera): Heliothis and Spodoptera.Annals of the Entomological Society of America68(2):371‑380. https://doi.org/10.1093/aesa/68.2.371
-
Silk weave and silk glands in aquatic instars of two species of Helicopsyche von Siebold, 1856 (Trichoptera, Helicopsychidae).Aquatic Insects22(1):58‑65. https://doi.org/10.1076/0165-0424(200001)22:1;1-z;ft058
-
The mechanism of digestion. In: Roeder KD (Ed.)Insect Physiology.John Wiley,New York,311-330pp.
-
Salivary glucose oxidase: Multifunctional roles for Helicoverpa zea?Archives of Insect Biochemistry and Physiology42(1):99‑109. https://doi.org/10.1002/(SICI)1520-6327(199909)42:1<99::AID-ARCH10>3.0.CO;2-B
-
Memoirs: Internal anatomy of a caddis (Hydropsyche colonica).Journal of Cell Science2(313):151‑179.
-
Über die Spinndrüsen der Lepidopteren.Wilhelm Engelmann
-
The structure of the salivary gland of the moth (Manduca sexta).Zeitschrift für Zellforschung und Mikroskopische Anatomie146(4):553‑564. https://doi.org/10.1007/bf02347183
-
Autophagy, apoptosis, and ecdysis-related gene expression in the silk gland of the silkworm (Bombyx mori) during metamorphosis.Canadian Journal of Zoology88(12):1169‑1178. https://doi.org/10.1139/Z10-083
-
Anatomy of the digestive system of Heliothis zea (Lepidoptera; Noctuidae) larvae.Bulletin - Mississippi Agricultural and Forestry Experiment Station905:1‑15.
-
Studies on the silk glands of the silkworm, Bombyx mori L. I. Morphological and functional studies of Filippi’s glands in the silkworm.Science Bulletin of the Faculty of Agriculture, Kyushu University22:95‑108.
-
Caterpillar saliva beats plant defences.Nature416(6881):599‑600. https://doi.org/10.1038/416599a
-
Evidence that the caterpillar salivary enzyme glucose oxidase provides herbivore offense in solanaceous plants.Archives of Insect Biochemistry and Physiology58(2):128‑137. https://doi.org/10.1002/arch.20039
-
Genomics of Lepidoptera saliva reveals function in herbivory.Current Opinion in Insect Science19:61‑69. https://doi.org/10.1016/j.cois.2017.01.002
-
NIH Image to ImageJ: 25 years of image analysis.Nature Methods9(7):671‑675. https://doi.org/10.1038/nmeth.2089
-
Insect silk glands: their types, development and function, and effects of environmental factors and morphogenetic hormones on them.International Journal of Insect Morphology and Embryology19(2):79‑132. https://doi.org/10.1016/0020-7322(90)90022-h
-
Structure and ultrastructure of the silk glands and spinneret of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae).Arthropod Structure & Development35(1):3‑13. https://doi.org/10.1016/j.asd.2005.10.002
-
Larval head anatomy of Heterogynis penella (Zygaenoidea, Heterogynidae), and a general discussion of caterpillar head structure (Insecta, Lepidoptera).Acta Zoologica86(3):167‑194. https://doi.org/10.1111/j.1463-6395.2005.00198.x
-
Ultrastructure of the Lyonet's glands in larvae of Diatraea saccharalis Fabricius (Lepidoptera: Pyralidae).Biocell28(2):165‑9.
-
Ultrastructure of Lyonet's gland in the silkworm (Bombyx mori L.).Journal of Morphology142(2):165‑185. https://doi.org/10.1002/jmor.1051420205
-
Comparative transcriptome analysis of Bombyx mori spinnerets and Filippi's glands suggests their role in silk fiber formation.Insect Biochemistry and Molecular Biology68:89‑99. https://doi.org/10.1016/j.ibmb.2015.11.003
-
The principles of insect physiology.Springer Science & Business Mediahttps://doi.org/10.1007/978-94-009-5973-6