|
Research Ideas and Outcomes : Project Report
|
|
Corresponding author: Mihael C Ichim (cichim@hotmail.com)
Received: 26 May 2018 | Published: 30 May 2018
© 2018 Mihael Ichim, Gianina Crisan, Carmen Tebrencu, Hugo de Boer
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation: Ichim M, Crisan G, Tebrencu C, de Boer H (2018) PhytoAuthent: Molecular authentication of complex herbal food supplements for safety and efficacy. Research Ideas and Outcomes 4: e26986. https://doi.org/10.3897/rio.4.e26986
|
|
Abstract
The PhytoAuthent project was structured to gather, test, develop and apply, in real life case scenarios, molecular techniques, such as biochemical fingerprinting and DNA sequence-based methods, for plant identification of constituents in complex herbal products. The project had a strong focus on applied aspects like protecting consumers from health risks associated with product substitution and contamination of herbal products.
Keywords
authentication, DNA barcoding, DNA metabarcoding, herbal products, medicinal plants
Introduction
The European Medicines Agency (EMA) is the sole responsible body for the evaluation of medicinal products in Europe, but with limited attributions related to herbal products because it lacks the regulations and the tools to uniformly monitor and control herbal products. The EMA supports the use of innovative analytical technologies, such as DNA barcoding, to complement traditional chromatographic identification methods for substitute, filler and adulterant detection in herbal products. Many herbal preparations and products have a history of safe use, but there is growing concern about their quality, efficacy and safety, as a result of studies showing major discrepancies between label information and analysed composition (
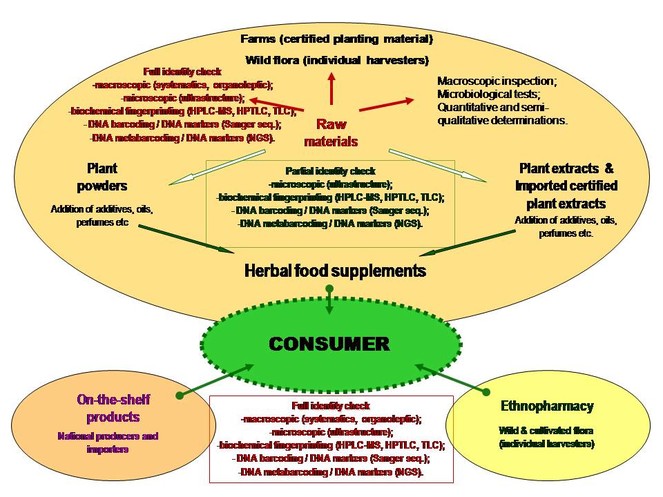
The PhytoAuthent project successfully addressed the ambiguity issues of the authenticity of herbal products (Fig.
Description
DNA metabarcoding was used to authenticate herbal products containing Echinacea (
In all our studies we focused on comparative methodological studies, where we compared DNA-based methods with biochemical analysis methods recommended by different pharmacopoeias for quality assessment, such as High Performance Liquid Chromatography coupled with Mass Spectrometry (HPLC-MS), High Performance Thin Layer Chromatography (HPTLC) and Thin Layer Chromatography (TLC). These biochemical fingerprinting methods, which have proved to be exact methods for authentication of the presence of target bioactive compounds of the analysed species, had limited effectiveness in the detection of substitution with related plant species and could not provide any information about other unknown ingredients of plant origin from the products.
Conclusions
If the safety of the herbal products is interpreted to the limit of specific bioactive compounds, then methods of chemical analysis are more relevant than analysis of the composition based on the DNA sequence data, but when analysing product authenticity, species substitution or falsification, DNA barcoding and DNA metabarcoding offer uncomparable resolution in species diversity detection (
Recommendations
Our results support the use of DNA based methods (DNA barcoding and metabarcoding) for the authentication of complex herbal products. The adoption of quality control standards by regulatory and control bodies could increase product quality and subsequently increase consumer confidence. Such standards would provide also an incentive for producers to increase internal quality control throughout the whole production chain, from plant raw materials and extracts to the final product.
Acknowledgements
The authors acknowledge the diligence, scientific expertise and commitment of all our colleagues from each of the four partner institutions.
Funding program
Romanian - EEA Research Programme operated by the MECS-ANCSI PO under the EEA Financial Mechanism 2009–2014 and project contract number 2SEE/2014.
Grant title
Molecular authentication of complex herbal food supplements for safety and efficacy (PhytoAuthent).
Hosting institution
National Institute of Research and Development for Biological Sciences (NIRDBS) / "Stejarul" Research Centre for Biological Sciences (Piatra Neamt, Romania).
Author contributions
MCI initiated the project. MCI, GC, CET and HdB designed the experiments and supervised their teams as Project Director (MCI) and Principal Investigators (GC, CET and HdB). MCI wrote the initial draft of the manuscript. MCI, GC, CET and HdB revised and reviewed the manuscript.
References
-
DNA barcoding and pharmacovigilance of herbal medicines.Drug Safety38(7):611‑620. https://doi.org/10.1007/s40264-015-0306-8
-
Veronica officinalis product authentication using DNA metabarcoding and HPLC-MS reveals widespread adulteration with Veronica chamaedrys.Frontiers in Pharmacology8:378. https://doi.org/10.3389/fphar.2017.00378
-
Comparative authentication of Hypericum perforatum herbal products using DNA metabarcoding, TLC and HPLC-MS.Scientific Reports7:1291. https://doi.org/10.1038/s41598-017-01389-w
-
Benefits and limitations of DNA barcoding and metabarcoding in herbal product authentication.Phytochemical Analysis29(2):123‑128. https://doi.org/10.1002/pca.2732
-
What’s in the box? Authentication of Echinacea herbal products using DNA metabarcoding and HPTLC.Phytomedicine33:32‑38. https://doi.org/10.1016/j.phymed.2018.03.058