|
Research Ideas and Outcomes :
Methods
|
|
Corresponding author: Paul Marek (paulemarek@gmail.com)
Received: 03 Jul 2017 | Published: 04 Jul 2017
© 2017 Paul Marek
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Marek P (2017) Ultraviolet-induced fluorescent imaging for millipede taxonomy. Research Ideas and Outcomes 3: e14850. https://doi.org/10.3897/rio.3.e14850
|
|
Abstract
Fluorescent imaging has been traditionally applied to cell biology, and more recently to entomology to capture microscopic images of insect anatomy. However, the technique has not been applied to the study of millipedes, most of which autofluoresce as a result of endogenous fluorescent molecules in their cuticle such as pterins and coproporphyrins. This study compares commercially available ultraviolet light sources for fluorescent photography of millipedes for the documentation of anatomical structures. Millipedes that were most strongly fluorescent were those in the order Polydesmida, and produced the brightest fluorescence that was most easily photographed using this technique. However, millipedes of the orders Spirobolida and Siphonophorida were also fluorescent and produced a bright blue visible emission. The best quality images were those obtained with a modified flash that produced the highest intensity and shortest wavelength ultraviolet light.
Keywords
Diplopoda, Motyxia, systematics, Xystodesmidae
Introduction
Millipede taxonomy has thrived with the introduction of modern laboratory techniques such as DNA sequencing, scanning electron microscopy, micro-computed tomography, and high depth of field photography. Fluorescence microscopy is one technique that has recently been introduced for visualizing and capturing morphological images in taxonomy. Fluorescence is an optical phenomenon whereby shorter wavelength light (typically ultraviolet) is absorbed by a material and re-emitted at a longer wavelength. The conversion takes place through relaxation of an excited singlet state orbital electron in a fluorescent molecule to a ground state thereby emitting a lower energy and longer wavelength photon (
During research on Motyxia species in California, and while photographing xystocheirine millipedes in the dark illuminated with handheld fluorescent lamps, the resulting images were frequently observed to be in sharp focus and striking in appearance due to the juxtaposition of the blue fluorescence against the dark background of the forest at night. The fluorescence of heavily calcified cuticle, antennae, legs and other regions with several layers of exoskeleton produced exceptionally sharp images. Since the heavily sclerotized gonopods are of primary interest in diplopod taxonomy, and are traditionally challenging to visualize due to their small size, experiments were conducted with several commercially available ultraviolet light sources and color filters to identify a suitable set of parameters for consistently taking high quality images of these features. In this study, I describe the experiments conducted to develop several inexpensive techniques for laboratory imaging of millipedes using UV induced fluorescence.
Materials and methods
Live specimens were collected and prepared according to
Photographs were taken with a Canon 6D dSLR camera with a 65 mm Canon MP-E macro lens mounted on a Passport II Portable Digital Imaging System (Canon, Tokyo, Japan; Visionary Digital, Charlottesville, USA). The Passport II includes a motor-based drive system to vary the focal distance for image stacking in Helicon Focus (HeliconSoft, Kharkiv, Ukraine). Images were captured at the shutter speed, aperture, and other settings described in Table
Illumination sources assessed in the study. Images of light sources shown in Suppl. material
|
Illumination source |
Maximum wavelength (nm) |
Comments |
Power, shutter speed, f-stop |
| Canon 199A modified flash, part: 199A | 350 | Modified stock flash. Images sharp, lacking purple or blue halos. Individual seta discernable. Dark background. Transparent, prostatic sperm groove visible. |
6V and required 4 AA batteries. Shutter: 1/125 s f-stop: 5.6 |
|
Halo UV-blacklight, 80 mm, part: AE80-UV48-BK |
450 |
80 mm diameter circular array of 48 SMD LEDs suspended 1 cm above the watch glass. Image of the gonopod was dominated by a blue halo concealing the outline of the setae. Medium transparency, prostatic sperm groove on apex somewhat visible. |
12V, no resistor required. Shutter: 2 s f-stop: 2.8 |
|
Halo UV-blacklight, 100 mm, part: AE100-UV33 |
450 |
100 mm diameter circular array of 33 SMD LEDs. Light suspended 6 cm above the watch glass. Gonopod image dominated with a purple halo concealing the outline of the seta. Medium transparency, prostatic sperm groove on apex somewhat visible. |
12V, no resistor required. Shutter: 1.5 s f-stop: 2.8 |
|
Three-array square SMD LED, part: LBM-UV 3SMB |
395 |
Three SMD LEDs arranged in a triangle. Light was oriented 6 cm from the specimen and at 30°. Gonopod image dominated with a blue halo that obscured setae. Several purple glare spots on the gonopod apex concealed the surface appearance. Lacking transparency. |
12V, no resistor required. Shutter: 2 s f-stop: 2.8 |
|
High power SMD LED, part: UV-1W-394 |
394 |
Light composed of a single high-power SMD LED requiring a heatsink. Prefemoral setae concealed behind purple halo. Broad spectrum light, produces some white light. Lacking transparency |
3.4V and required a resistor and heatsink. Shutter: 1.5 s f-stop: 2.8 |
|
High power SMD LED starlight, part: PG1C-1LLS-Q3 |
400 |
Light composed of a single high-power SMD LED requiring a heatsink. Contacts difficult to solder. Quickly smoked and melted without capturing an image. |
3.5V and required a resistor and heatsink. Shutter: N/A f-stop: N/A |
|
5 mm LED, part: RL5-UV0315-380 |
380 |
Light composed of a single 5 mm LED. Prefemoral setae concealed behind dark purple halo. Medium transparency, prostatic groove on apex somewhat visible. |
3.5V and required a resistor. Shutter: 1 s f-stop: 2.8 |
|
5 mm LED, radial, part: RL5-UV0315-380 |
380 |
Light composed of three 5-mm LEDs. Image of the gonopod was dominated by a purple background and halo concealing the outline of the setae. Medium transparency, prostatic sperm groove on apex somewhat visible. |
12V, no resistor required. Shutter: 1 s f-stop: 3.5 |
|
5 mm LED, part: RL5-UV0430-400 |
400 |
Light composed of a single 5 mm LED. Gonopod image dominated with a purple halo that obscured setae. Several purple glare spots on the gonopod concealed the surface appearance. Lacking transparency. |
3.5V and required a resistor. Shutter: 2 s f-stop: 2.8 |
Ultraviolet light sources were assessed for their ability to capture a fluorescent image of millipede structures. Images were qualitatively assessed by visual examination and favored for a small aperture and a short exposure time. Ultraviolet light-emitting diodes (LEDs) and the modified Canon flash were compared. Since camera flashes typically possess a filter that blocks ultraviolet light, the stock flash was modified to permit short wavelength light between 290 – 360 nm to pass. The Canon 199A has a plastic UV-filter in front of the flash bulb that was easily removed by unscrewing two small screws at the base and unclipping the plastic filter from its housing (
Results
Images taken with the Canon 199A flash are shown below and results summarized in following descriptions.
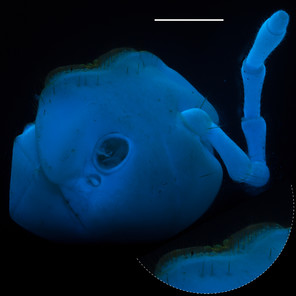
Motyxia tularea tularea Xystodesmidae (Fig.
Motyxia sequoiae, Xystodesmidae (Fig.
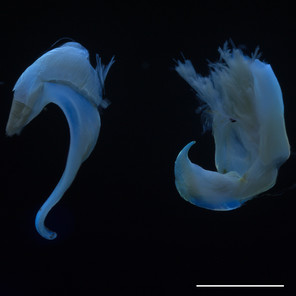
Fluorescent images of millipedes imaged in 350 nm UV light with a Canon 199A modified flash. Scale bars = 1 mm.
b: Head and anterior trunk rings of Siphonacme lyttoni
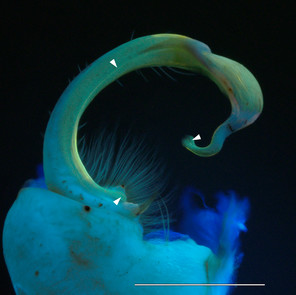
c: Foreleg of male Pseudopolydesmus canadensis, inset: striated muscles (top) and sphaerotrichomes (bottom)
d: Posterior (left) and anterior (right) gonopods of Tylobolus uncigerus
Siphonacme lyttoni, Siphonophoridae (Fig.
Tylobolus uncigerus, Spirobolidae (Fig.
Pseudopolydesmus canadensis, Polydesmidae (Figs
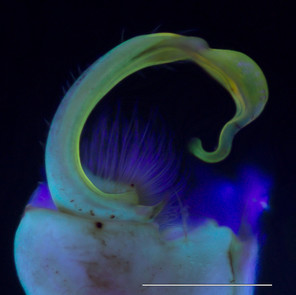
Brachoria sheari, Xystodesmidae (Fig.
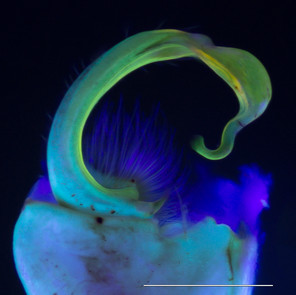
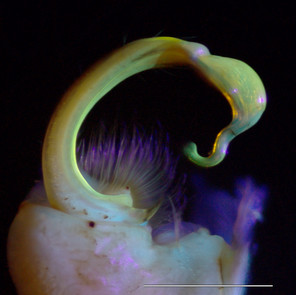
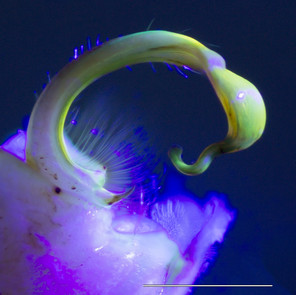
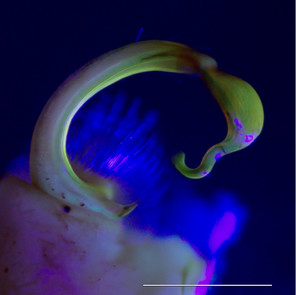
Images of the left gonopod from a male Brachoria sheari illuminated with different light sources. Scale bar = 1 mm. Prostatic sperm groove labeled with arrows.
b: Incident visible (white) light
c: 5 mm 380 nm UV LED radial light
d: 100 mm halo UV light
e: 80 mm halo UV light
f: 5 mm 380 nm UV LED light
The images of the B. sheari gonopod photographed with the commercially available UV lights are shown in Figs
Discussion
The ease of UV induced visible light fluorescence photography is perhaps due to the bright fluorescent emission that is dominant in green wavelengths, to which digital camera sensors are particularly sensitive—green-sensitive elements outnumber blue and red by a factor of two on the Bayer filter mosaics ubiquitous in digital cameras (
Every millipede imaged with UV illumination was fluorescent. The Polydesmida, and in particular Motyxia and Xystodesmidae, were strongly fluorescent. The images of the gonopod and leg of the polydesmid species P. canadensis were sharp and noteworthy because they contain structures that were delineated with different hues. The colors range from blue to green, and the medial processes and pulvillus appeared much sharper compared to the image captured with visible light (Fig.
Conclusions
Fluorescent imaging may provide a useful and inexpensive method for capturing high quality images for millipede taxonomy. Using the endogenous fluorescence of millipedes, subtle structures such as the prostatic sperm groove are noticeable and ultraviolet illumination preserves transparency such that small spines and processes—even muscle fibers in some cases—can be seen through cuticle that may otherwise obscure it. The technique allows examination of fine shapes from different angles, more than may be possible with visible light or scanning electron micrography.
Though many research institutions possess confocal microscopes, and these instruments have been successfully demonstrated to capture arthropod cuticle morphology, there may be an hourly charge for beam time and a queue if the instrument is frequently used. This study concludes that four portable inexpensive ultraviolet light sources work well for fluorescent imaging. (1) The two circular halo lights and the 380 nm 5-mm LEDs arranged in a radial pattern generated sharp evenly fluorescent images (Fig.
Acknowledgements
Petra Sierwald provided comments and revisions on previous versions of the manuscript.
Funding program
This study was supported by a NSF Systematics and Biodiversity Science Advancing Revisionary Taxonomy and Systematics (ARTS) award to P. Marek (DEB# 1655635). Supplemental funding was provided by a Virginia Tech USDA NIFA Hatch Project (VA-160028).
Conflicts of interest
The author states there is no conflict of interest.
References
- Color imaging array.Google Patents
- The Optics of Life.Princeton University Press,Princeton,336pp. https://doi.org/10.1515/9781400840663
- Identification of a fluorescent compound in the cuticle of the train millipede Parafontaria laminata armigera.Bioscience, biotechnology, and biochemistry74(11):2307‑2309. https://doi.org/10.1271/bbb.100171
- 7,8-Dihydropterin-6-carboxylic acid as light emitter of luminous millipede, Luminodesmus sequoiae.Bioorganic & Medicinal Chemistry Letters11(8):1037‑1040. https://doi.org/10.1016/s0960-894x(01)00122-6
- Use of confocal laser scanning microscopy in systematics of insects with a comparison of fluorescence from different stains.Systematic Entomology34(1):10‑14. https://doi.org/10.1111/j.1365-3113.2008.00451.x
- Discovery of a glowing millipede in California and the gradual evolution of bioluminescence in Diplopoda.Proceedings of the National Academy of Sciences112(20):6419‑6424. https://doi.org/10.1073/pnas.1500014112
- Bioluminescent aposematism in millipedes.Current Biology21(18):R680‑R681. https://doi.org/10.1016/j.cub.2011.08.012
- Fluorescence as a means of colour signal enhancement.Philosophical Transactions of the Royal Society B: Biological Sciences372(1724):20160335. https://doi.org/10.1098/rstb.2016.0335
- A general methodology for collecting and preserving xystodesmid and other large millipedes for biodiversity research.Biodiversity Data Journal3:e5665. https://doi.org/10.3897/bdj.3.e5665
- UVIROPTICS: Specialty infrared and ultraviolet camera filters. www.etsy.com/uviroptics. Accessed on: 2017-6-28.
- Integumental Pigments of the Millipede, Polydesmus angustus (Latzel).Nature217(5132):975‑977. https://doi.org/10.1038/217975a0
- The terrestrial bioluminescent animals of Japan.Zoological science28(11):771‑789. https://doi.org/10.2108/zsj.28.771
- Selected least studied but not forgotten bioluminescent systems.Photochemistry and Photobiology93(2):405‑415. https://doi.org/10.1111/php.12704
- The cyanide gland of the greenhouse millipede, Oxidus gracilis (Polydesmida: Paradoxosomatidae).Research Ideas and Outcomes3:e12249. https://doi.org/10.3897/rio.3.e12249
- Luminescent myriapoda: a brief review.Bioluminescence in Focus: A Collection of Illuminating Essays.Research Signpost,Trivandrum, India,385pp. [ISBN978-81-308-0357-9].
- UV Blacklight Photography Tutorial (Ultraviolet-Induced Visible Fluorescence. http://photoextremist.com/ultraviolet-induced-visible-fluorescence-photography-tutorial. Accessed on: 2017-6-28.
- A re-evaluation of the milliped genus Motyxia Chamberlin, with a re-diagnosis of the tribe Xystocheirini and remarks on the bioluminescence (Polydesmida: Xystodesmidae).Insecta Mundi11:331‑351. URL: http://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1279&context=insectamundi
- Bioluminescence - Chemical Principles and Methods.World Scientific,Hackensack. https://doi.org/10.1142/9789812773647
Supplementary material
LED lights assessed in this study. A. 5 mm 380 nm LED light; B. 5 mm LED radial light; C. SMD LED starlight; D. SMD triple UV light; E. SMD single LED light; F. Canon 199A 350 nm UV light; G. 5 mm LED radial light; H. 80 mm halo UV light; I. 100 mm halo UV light.