|
Research Ideas and Outcomes : Case Study
|
|
Corresponding author: István Mikó (istvan.miko@gmail.com)
Received: 13 Feb 2017 | Published: 14 Feb 2017
© 2017 Kirsten Pearsons, István Mikó, John Tooker
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation: Pearsons K, Mikó I, Tooker J (2017) The cyanide gland of the greenhouse millipede, Oxidus gracilis (Polydesmida: Paradoxosomatidae). Research Ideas and Outcomes 3: e12249. https://doi.org/10.3897/rio.3.e12249
|

|
Abstract
Although the greenhouse millipede, Oxidus gracilis, is distributed worldwide, there is little work using modern tools to explore its morphology. We used confocal laser scanning microscopy (CLSM) to image the cyanide glands of Oxidus gracilis. Glands from adult millipedes were dissected out before imaging, and we were able to image glands of juveniles through the cuticle due to the strong autofluorescence of the gland extract. We can report that CLSM is a promising technique to non-invasively investigate the development and mechanisms of polydesmid cyanide glands.
Keywords
Oxidus gracilis, cyanide gland, Diplopoda, Polydesmida, CLSM
Background
The following work is the result of the "Know Your Insect" graduate course in the Department of Entomology at the Pennsylvania State University, taught during the fall semester of 2016. In short, the course provides the opportunity for a small group of students to learn more about the insects they are studying. Each student focuses on an anatomical feature of interest and leads the class in a lecture, discussion, and live dissection. The students also have the opportunity to image their anatomical feature of interest using confocal laser scanning microscopy (CLSM), relying on the autofluorescence of arthropod tissue (
Through work on decomposer communities in Pennsylvanian agricultural fields, KP came across the greenhouse millipede, Oxidus gracilis. O. gracilis was the only millipede species she collected in corn and soybean fields in the summer of 2016, and they dominated her samples (over 35% of macroinvertebrates collected in pitfall traps). Because O. gracilis is an invasive species, KP was curious to learn more about their invasion, the morphological and ecological mechanisms that allow them to reach such high densities, and implications of their presence for decomposition and nutrient cycling.
Overall KP's case was unique for this course, as her "insect" is not an insect at all but rather a diplopod; while working with a diplopod provided some challenges, we encountered some exciting surprises.
New information
As with many other arthropod groups, interest in studying millipede morphology has varied over time. Overall, diplopods have received little attention compared to other arthropod groups (
We are also excited to report that, at least up to the 5th larval stadium, the cuticle is transparent enough to image the highly auto-fluorescing storage chamber without dissection. We believe that non-invasive imaging of the glands has multiple potential applications; it could be used to do live imaging of when glands are activated, to observe the growth and development of the glands across stadia, and to determine the amount of defensive secretions released under different stressors.
During dissection of the adult specimens, we noticed heavy infestation nematodes. It would be interesting to know if these nematodes act as a significant top-down control on O. gracilis populations, although high millipede densities in our sample site would lead us to hypothesize they have little control.
Overview of the cyanide glands in millipedes
Cyanide glands (=repugnatorial glands,
Cyanide glands are usually present in just over half the body segments - 5, 7, 9, 10, 12, 13, 15-19 - and are located in the paired, flange-like pleural keels (
Methodology
Field collection and rearing of Oxidus gracilis
We collected adult Oxidus gracilis millipedes from a corn field at the Russell E. Larson Agricultural Research Center at Rock Springs, in Centre County Pennsylvania, USA, during summer and and fall of 2016.
After summer storms, moist soil and high humidity provided for easy surface collection of adult Oxidus gracilis. We collected approximately 100 adults on 28 July 2016; once in the lab, we kept the millipedes in a clear plastic container with a 1-2 cm layer of field soil and a handful of moistened straw. We maintained the colony of 100 adults for four weeks; after three weeks (18 August 2016) we observed clusters of eggs and second instar larvae. However, it was difficult to maintain the colony under proper humidity - too dry and the colony desiccated, too wet and fungus overgrew the colony. The majority of the colony was lost by mid-September; interestingly, this coincided when adult field populations began to decline. In their hypothesized native range of Japan, Oxidus gracilis has been observed to have a similar life history - millipedes mature in summer, lay eggs in fall, and overwinter as late larval stages (
Specimen preparation and dissection
Adult specimens collected in September were fixed in 80% ethanol. Upon dehydration in ethanol, the cyanide gland became impossible for us to remove – likely due to the gland tissue collapsing. Because of this observation, adult specimens collected in October were kept alive until dissection. At the time of dissection, we placed the millipedes in a petri dish containing 0.1 M dibasic phosphate buffer (ph=7.4) and quickly severed the head from the body using dissecting scissors. Timing of this process seems to be critical; if the millipede is disturbed too much before the dissection, the glands’ storage chambers may be emptied which makes it hard to see the clear gland tissue against the fat body.
After decapitation, the body rings were carefully separated from each other and from the digestive system. Only the segments containing cyanide glands (rings 5, 7, 9, 10, 12, 13, and 15-19) were dissected further under light microscopy; before each ring dissection we checked for the gland ozopore on each keel to ensure we were dissecting rings with cyanide glands (Suppl. material
For each ring dissection, the dorsal tergite and ventral sternite were bisected so each keel could be dissected independently. Care was taken to remove fat body and cuticle to reveal the storage and reaction chambers of each gland.
We use two, fifth stadium juvenile millipedes for additional imaging. Noticing how transparent the cuticle was, and knowing how fluorescent the storage chambers were from the adult dissections, we did not attempt to fully dissect out the glands. From one juvenile we isolated body rings with ozopores and we left the other juvenile largely intact, only severing the head to reduce movement.
Confocal Laser Scanning Microscopy
Specimens were examined with an Olympus FV10i Confocal Laser Scanning Microscope using two excitation wavelengths: 473 nm, and 559 nm. We detected autofluorescence using two channels with emission ranges of 490–590 nm (green pseudocolor), and 570–670 nm (red pseudocolor), respectively. We generated volume rendered micrographs and media files with ImageJ (
Observations on the cyanide glands using dissections and Confocal Laser Scanning Microscopy
Adults
Some glands were easily identified under a light microscope by a characteristic brownish-yellow droplet suspended within the storage chamber. Droplets like this have been observed across polydesmids and are presumed to be the precursor mandelonitrile (
Most storage chambers were difficult to see or not found at all, likely due to destruction of the delicate storage chambers during dissection, or the millipedes evacuating their storage chambers upon disturbance.
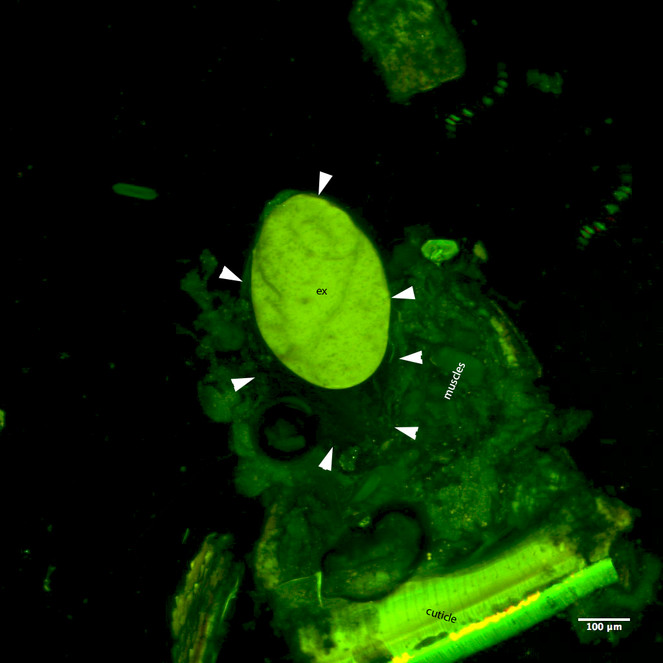
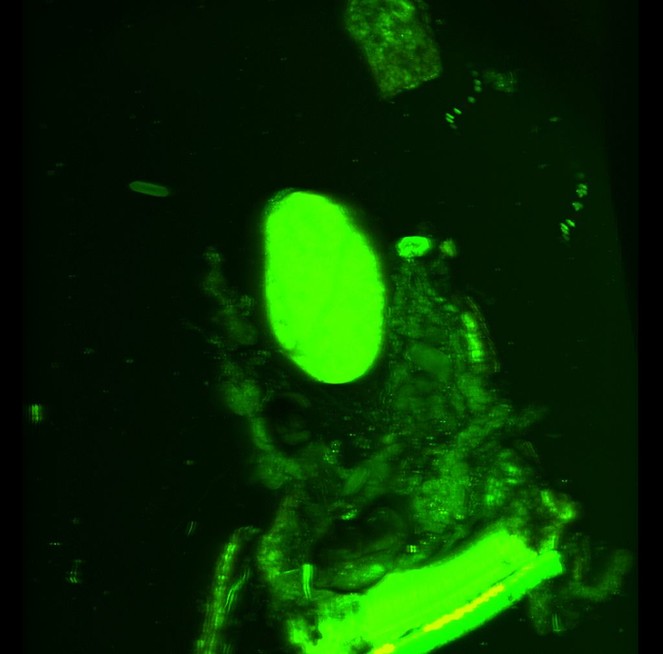
We successfully dissected out a storage chamber from one cyanide gland which we imaged on the CLSM using autofluorescense (Figs
CLSM volume rendered micrograph showing the cyanide gland of Oxidus gracilis (arrows pointing the wall of the cyanide gland, ex=strongly autofluorescing gland extract).
Juveniles
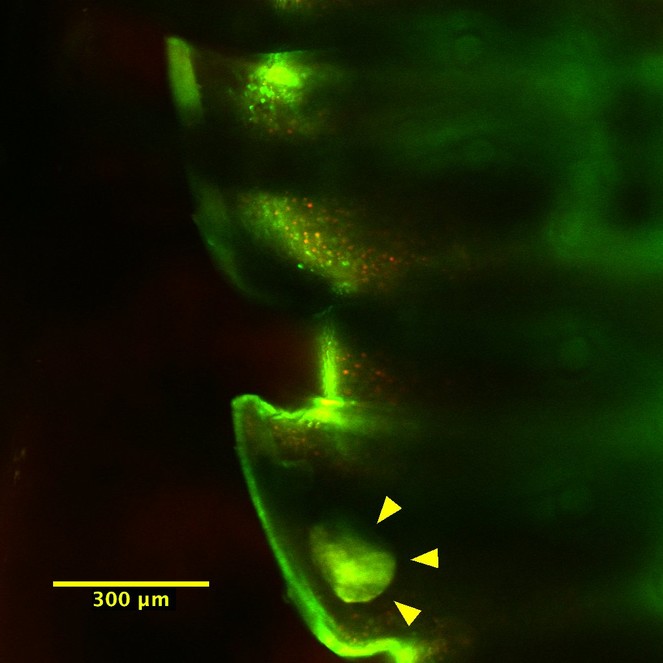
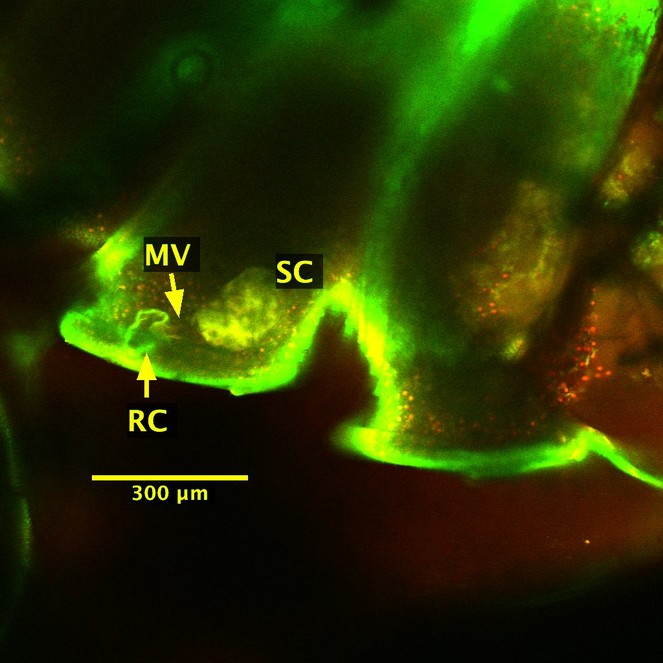
We were unable to observe the cyanide glands from the dissected juvenile millipede; it is possible the gland structures are even more delicate in juveniles, and therefore more prone to tearing during dissection. However, because the cuticle is transparent in the 5th larval stadium, we successfully imaged the strongly fluorescing storage chambers through the cuticle of the intact juvenile millipede (Figs
Top view of the juvenile millipede; the bright field in the lower flange is a gland storage chamber.
Nematode infestation in adults
Upon dissection, we observed some of the adult specimens contained numerous nematodes (Suppl. material
Relevance to ongoing research
One of us (KP) is studying the effects of insecticides on macrodecomposer activity in corn and soybean fields in Pennsylvania. Before this field season, her advisor mentioned the prevalence of millipedes in some fields, and her fieldwork this summer confirmed this – over 35% of specimens in pitfall traps (in a maize and soy field at our University’s research farm) were millipedes (KP, pers. obs.). Of these, virtually all of them were Oxidus gracilis. With such a dominant millipede species, we were interested, although not entirely surprised, to discover it was an invasive species.
Invasive decomposers are prevalent – invasive earthworms are often dominate agricultural and forest decomposer communities throughout the United States (
Decomposer food web
Of course, agricultural systems aren't pristine – they've been invaded by both destructive species (slugs and other crop pests) and beneficial species (carabid beetles, earthworms). We are interested in how O. gracilis fits into this novel food web - how do they interact with pests, predators, and other decomposers? Some farmers worry that the millipedes may be damaging their crops, although O. gracilis refused to eat live plants in laboratory experiments (
We chose to image the cyanide glands of Oxidus gracilis because the defenses of this invasive species potentially tie into their interactions with generalist predators found in central Pennsylvania. Around 90% of plant biomass is recycled through decomposers, meaning the bulk of plant-based energy travels to predators not through herbivores, but through decomposers (
If decomposers are acceptable, but less desirable prey, they can sustain predator populations when pest pressures are low, but not interfere with control when pest populations increase. Unfortunately, it is difficult to predict how valuable decomposers are for pest management because interactions between generalist predators and decomposers are poorly documented. The generalist predator community in Pennsylvania field crops is dominated by carabid beetles and spiders. Some carabid beetles reject earthworms and isopods (
Acknowledgements
We thank Loren Rivera-Vega, Bipana Paudel, Ryan Reynolds, Anne Jones, Asher Jones and Samita Limbu for their help in dissections and for useful discussions during the course of this work and Missy Hazen (Penn State Microscopy and Cytometry Facility - University Park, PA) for her help with CLSM.
References
-
Collembola as alternative prey sustaining spiders in arable ecosystems: prey detection within predators using molecular markers.Molecular Ecology12(12):3467‑3475. https://doi.org/10.1046/j.1365-294X.2003.02014.x
-
Brown Ground: A Soil Carbon Analogue for the Green World Hypothesis?The American Naturalist167(5):619‑627. https://doi.org/10.1086/503443
-
Introduced earthworms in agricultural and reclaimed land: their ecology and influences on soil properties, plant production and other soil biota.Biological Invasions8(6):1301‑1316. https://doi.org/10.1007/s10530-006-9024-6
-
Food Preferences of Five Species of Carabids Commonly Found in Iowa Cornfields.Environmental Entomology6(1):9‑12. https://doi.org/10.1093/ee/6.1.9
-
Generalist Predators (Coleoptera: Carabidae, Staphylinidae) Associated With Millipede Populations in Sweet Potato and Carrot Fields and Implications for Millipede Management.Environmental Entomology38(4):1106‑1116. https://doi.org/10.1603/022.038.0418
-
Studies on the Life History and the Ecology of the Hothouse Millipede, Orthomorpha gracilis (C. L. Koch 1847).American Midland Naturalist29(3):670. https://doi.org/10.2307/2421156
-
Cyanide and arthropods.Cyanide in Biology.
-
Benzoyl cyanide and mandelonitrile benzoate in the defensive secretions of millipedes.Journal of Chemical Ecology3(1):101‑113. https://doi.org/10.1007/bf00988137
-
Cyanogenic Glandular Apparatus of a Millipede.Science139(3560):1218‑1220. https://doi.org/10.1126/science.139.3560.1218
-
Autofluorescence imaging, an excellent tool for comparative morphology: AUTOFLUORESCENCE IMAGING.Journal of Microscopy244(3):259‑272. https://doi.org/10.1111/j.1365-2818.2011.03534.x
-
The Impact of Invasive Earthworms on Soil Respiration and Soil Carbon Within Temperate Hardwood Forests.Ecosystems19:942. https://doi.org/10.1007/s10021-016-9977-y
-
The Marek Lab. Cyanide gland dissections. http://jointedlegs.org/?p=192. Accession date: 2016 12 31.
-
Discovery of a glowing millipede in California and the gradual evolution of bioluminescence in Diplopoda.Proceedings of the National Academy of Sciences112(20):6419‑6424. https://doi.org/10.1073/pnas.1500014112
-
Treatise on Zoology - Anatomy, Taxonomy, Biology. The Myriapoda, Volume 2.Brill,482pp. URL: http://dx.doi.org/10.1163/9789004188273 [ISBN978-90-04-18827-3] https://doi.org/10.1163/9789004188273
-
Postembryonic development of the common myriapoda of Japan X. Life history of Oxidus gracilis (KOCH)(Diplopoda, Strongylosomidae).Zoological Magazine71.
-
A correction regarding the article of the life history of Oxidus gracilis Koch.Zoological Magazine75.
-
Effect of neoaplectanid and heterorhabitid nematodes (Nematoda: Rhabditoidea) on the millipede Oxidus gracilis.Journal of invertebrate pathology45(2):231‑235. https://doi.org/10.1016/0022-2011(85)90013-8
-
Food web complexity and community dynamics.American Naturalist147:813‑846. https://doi.org/10.1086/285880
-
Invasive Earthworms Deplete Key Soil Inorganic Nutrients (Ca, Mg, K, and P) in a Northern Hardwood Forest.Ecosystems18(1):89‑102. https://doi.org/10.1007/s10021-014-9814-0
-
NIH Image to ImageJ: 25 years of image analysis.Nature Methods9(7):671‑675. https://doi.org/10.1038/nmeth.2089
-
The chemical defenses of millipedes (diplopoda): Biochemistry, physiology and ecology.Biochemical Systematics and Ecology61:78‑117. https://doi.org/10.1016/j.bse.2015.04.033
-
Current Status of the Myriapod Class Diplopoda (Millipedes): Taxonomic Diversity and Phylogeny.Annual Review of Entomology52(1): . https://doi.org/10.1146/annurev.ento.52.111805.090210
-
Earthworm invasion in North America: Food resource competition affects native millipede survival and invasive earthworm reproduction.Soil Biology and Biochemistry57:212‑216. https://doi.org/10.1016/j.soilbio.2012.08.022
-
Do earthworms help to sustain the slug predator Pterostichus melanarius (Coleoptera: Carabidae) within crops? Investigations using monoclonal antibodies.Molecular Ecology9(9):1279‑1292. https://doi.org/10.1046/j.1365-294x.2000.01006.x
-
Identification of secretory compounds from the millipede, Oxidus gracilis C. L. Koch (Polydesmida: Paradoxosomatidae) and their variation in different habitats.Applied Entomology and Zoology38(3):401‑404. https://doi.org/10.1303/aez.2003.401
-
Linking the green and brown worlds: the prevalence and effect of multichannel feeding in food webs.Ecology95(12):3376‑3386. https://doi.org/10.1890/13-1721.1
Supplementary material
Download file (560.75 kb)