|
Research Ideas and Outcomes : PostDoc Project Plan
|
|
Corresponding author: Jens Joschinski (jens.joschinski@uni-wuerzburg.de)
Received: 16 Jun 2016 | Published: 17 Jun 2016
© 2016 Jens Joschinski.
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation: Joschinski J (2016) Benefits and costs of aphid phenological bet-hedging strategies. Research Ideas and Outcomes 2: e9580. doi: 10.3897/rio.2.e9580
|

|
Abstract
Intended readership
I am looking for a host institute to research bet-hedging strategies in the seasonal reproductive mode switches of aphids. The intended methods leave room for collaborative side-projects beyond the study question (e.g. molecular control of photoperiodism, or sharing aphid lines from throughout Europe), so this article might be of interest to anyone working with aphids. In addition, I would be happy to receive feedback from experts in bet-hedging theory, phenotypic plasticity and photoperiodism.
Summary
Global change causes both mean temperature and temperature variability to increase. Organisms can cope with predictable change in means, but increasing variability is less tractable. One strategy to increase survival chances in unpredictable environments is diversified bet-hedging, i.e. spreading the risk by variation in phenotype expression. Despite being considered a general mechanism, definite evidence for bet-hedging is scarce, as it requires both the demonstration that phenotypic variance correlates with environmental variability, and that it maximizes fitness.
When assessing fitness, one needs to account for both the benefits and costs of bet-hedging. Bet-hedgers produce suboptimal phenotypes in average years, resulting in decreased arithmetic mean fitness. But this temporary reduction is more than compensated by elevated fitness in harsh years, so this well-known short-term fitness effect is not considered a real cost of bet-hedging. In contrast to the effects on arithmetic mean fitness, I hypothesize that bet-hedging also carries a long-term (geometric mean) fitness costs, in that the ability to generate phenotypic variance is costly per se.
With this research idea I seek evidence for bet-hedging and assess its costs and benefits, using aphids and their polyphenism in reproductive modes as model system. I plan to use aphid clones from environments along a gradient of temperature variability, and induce switches in reproductive modes under controlled conditions. To test for bet-hedging, I will correlate variance in phenotype determination with variability of the original environment. To determine the costs of bet-hedging, I will compare population growth of bet-hedgers with non-hedging clones. I will then combine benefits and costs of bet-hedging by calculating the geometric (long-term) mean fitness in predictable and unpredictable environments.
Keywords
bet-hedging; stochastic polyphenism; fluctuating natural selection; phenotypic plasticity; phenology; photoperiodism; circadian clocks; aphids; Acyrthosiphon pisum; Rhopalosiphum padi; Megoura viciae
Objectives, Concept and Approach
Climate change is the largest challenge of the 21st century (
When climate change drives environmental parameters out of tolerable limits, survival is generally considered to depend on microevolution and phenotypic plasticity (
Bet-hedging is adaptive variance of phenotypes around an ‘optimal’ mean value that can buffer against unforeseen environments. For example, variability in seed germination ensures that not all seeds enter the vulnerable seedling stage at the same time, so dormant seeds can survive to the next year under adverse conditions (
Costs and limits of bet-hedging
Why is evidence for bet-hedging so scarce? On the one hand, the prevalence of bet-hedging might be underestimated due to the challenge of finding good evidence (
According to the conceptual framework, true costs of plasticity need to be separated from phenotype-environment mismatches. A plastic organism that is imperfectly matched to the environment is still better adapted than a canalized organism, which does not fit at all to one environment. Thus, mismatches with the environment represent the limit of an adaptive strategy, and cannot be considered true costs of plasticity. The real costs of phenotypic plasticity can then be split into global (maintenance) and local (production) costs. Maintenance costs are independent of the environment, and arise from the ability to be plastic per se, e.g., having the machinery to detect environmental change. Production costs, on the other hand, arise only when phenotypic plasticity is expressed. I argue that this discussion on costs and limits is very similar for bet-hedging.
In accordance with the discussion on phenotypic plasticity, true costs of bet-hedging need to be separated from limits of the otherwise adaptive strategy: By inducing sub-optimal phenotypes, bet-hedging decreases the fitness in an average environment. This decrease in arithmetic mean fitness is not a true cost, but the core of the insurance strategy that increases fitness over longer times, i.e., geometric mean fitness (
Similar to costs of phenotypic plasticity, costs of bet-hedging can be split into global (maintenance) and local (production) costs. I hypothesize that maintenance costs manifest in a lower overall fitness, because bet-hedging requires a mechanism to generate variability. Bet-hedging could for example be achieved by high sensitivity to small environmental change (microplasticity), as has been observed in the response of seeds to germination conditions (
Aphids as models for bet-hedging
One possible bet-hedging mechanism is stochastic polyphenism, i.e. a stochastic choice of alternative phenotypes (
Project description
With this research idea I want to ask whether seasonal polyphenism is a bet-hedging strategy, and whether its evolution is hampered by fitness costs. I am looking for collaborations, as well as a working environment and research team for a postdoctoral project.
Aphids reproduce asexually over summer, and switch to production of sexual forms when the days shorten. The response to day length follows a logistic curve (
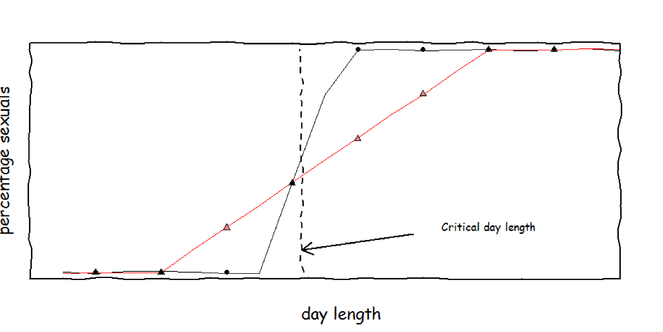
Phenotypic variance in sexual offspring production. When aphids are subjected to different day lengths, the induction of sexual offspring follows a logistic curve. Near the critical day length (were 50% of the offspring are sexual) there is a transient period in which the choice of offspring type is stochastic. I expect that clones from predictable (black, circles) and unpredictable (red, triangles) environments differ in slope of day length response and thus in the extent of the transient period. In total, 12 clones, each from a different environment, will be tested.
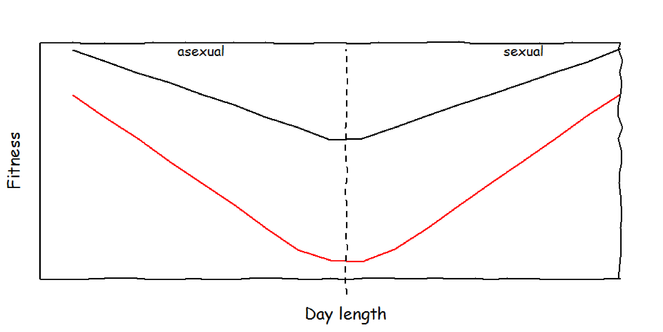
Maintenance and production costs of bet-hedging. I expect that aphids which invest into bet-hedging produce offspring of lower quality, irrespective of environmental conditions (maintenance costs). In addition, I expect that the costs increase when the bet-hedging trait is expressed (production costs). To test these hypotheses, the data will be analysed separately for sexual and parthenogenetic parents (dashed line = critical day length).
Implementation
Below I propose one potential research plan for the main experiment. Methodology, choice of species and origin of aphid clones are all amendable to change, as they depend on the infrastructure and location of the host institute, on funding and on further collaborations. I first assume that I will collect Acyrthosiphon pisum from various locations of Europe, and that the experiment will be split into four smaller experiments with two day length treatments each (for a total of eight day lengths) to accommodate for space limitations. I will then address potential variations of the methodology in the next section.
Methodology
Origin of clones
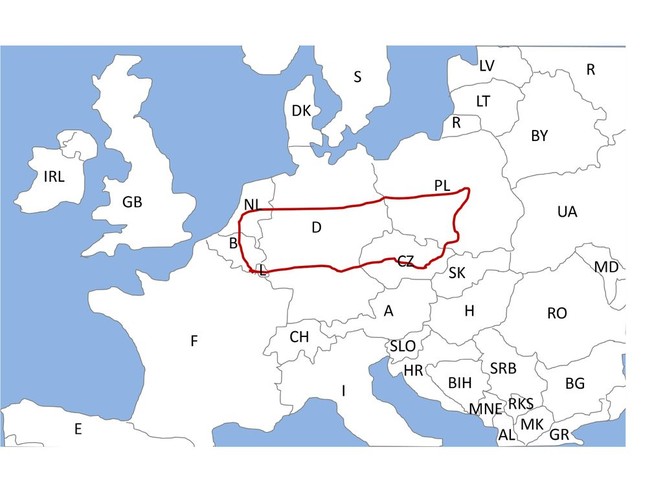
To obtain aphids with sufficiently different environmental and genetic backgrounds, I require aphid lines originating from a larger regional scale. Nevertheless, to compare the spread of reproductive mode switch in a single experiment, the different lines need to be induced at a similar mean (critical) day length, which correlates with latitude (
Climate data
The experiment requires aphid lines from a gradient of winter unpredictability. This has been defined by
While the standard deviation in onset of winter can be easily calculated, it neglects adaptive phenotypic plasticity. For example, the response to day length is modulated by temperature (
Experiment
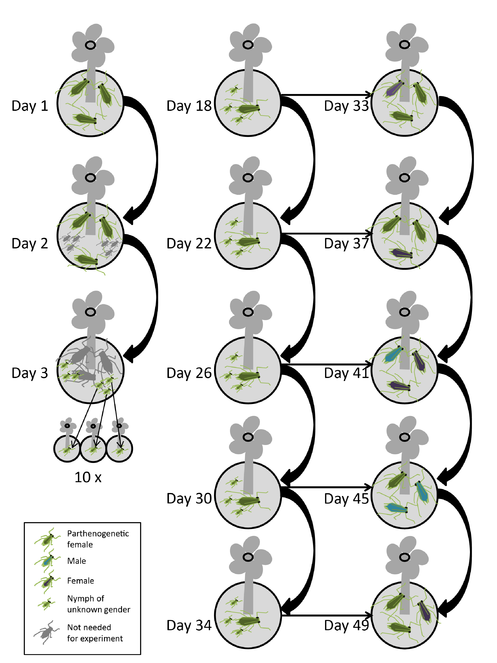
The procedure for 12 clones kept at two day lengths is as follows (
Hypothesis 1: variance in reproductive modes correlates with winter unpredictability
When plotting the percentage of sexual offspring against day length, I will obtain a logistic curve as in
glmer (Induction ~ treatment * environment + (1 | mother/environment), family = binomial)
A significant interaction will support the hypothesis that the transient period correlates with environmental unpredictability. In addition, a significant positive effect of environment alone would indicate that clones from the most unpredictable environments also take riskier (later) strategies, as the modelling approach of
Bet-hedging could be achieved by two different modes: First, a single mother aphid can produce both sexual and asexual offspring. Secondly, each mother can produce only one type of offspring, but the choice of the type differs among genetically identical mothers. Literature suggests that mixed families exist in A.pisum (
Hypothesis 2: Bet-hedgers suffer maintenance and production costs
Because photoperiodic induction requires rearing all offspring I will obtain detailed data on life-history traits. I hypothesize that clones which cope with high environmental unpredictability suffer from generally reduced fertility (maintenance costs), or from higher production costs when reared near the critical day length that induces transition from asexual to sexual reproduction (
Because development times and fecundity per day of the parent aphids are known, I construct life history tables and derive population growth based on a Leslie matrix, which gives a detailed account of aphid fitness (
For both dataset I will apply the model:
Fitness ~ day_length * environment + (1 | mother/environment)
A significant interaction of day length and environment is evidence for production costs (increased costs close to critical day length). If there is a significant effect of environment, this will be evidence for maintenance costs (generally reduced fitness).
Hypothesis 3: The degree of bet-hedging matches quantitatively with environmental variance
Strong evidence for bet-hedging is provided by the observation that phenotypic variance can be quantitatively predicted by environmental variation (
Adaptations
Depending on host institute and collaborative projects, several details of the experiment can be adapted. Below I describe potential variations from the research plan.
Species
Because polyphenism in reproductive modes is a primitive feature of the Aphidoidea (
A.pisum is an emerging model organism in ecology (
Origin of clones
The experiment requires aphid lines from several locations throughout Europe. I plan to sample aphid lines shortly before the experiments, because aphids can evolve quickly despite being asexual (
Logistics
The experiment is logistically challenging. It requires frequently transferring aphids from 240 plants to new plants, and I need 1200 host plants in total. The procedure would be considerably easier, if the parent’s reproductive mode was known without having to rear all offspring. If the sexuality of the offspring could be determined on newly born nymphs, it would reduce the number of plants to 240 and reduce the time from 49 to 34 days. Thus, I would be happy for any suggestions to determine reproductive modes of young offspring morphologically.
In A. pisum, the reproductive mode of the parents could also be determined by analysing gene expression of the young offspring (
Location
I am looking for collaborators with knowledge in aphid biology and/or bet-hedging theory, and for a research environment that has at least two climate chambers and sufficient greenhouse space to rear about 1200 plants. The project will require approximately 3000€ to collect aphids, as well as small funds for aphid and plant rearing, and for student helpers for general maintenance of aphid clones and plants.
Further considerations
The proposed research project carries the danger that aphids do not hedge their bets, which makes an analysis of costs of bet-hedging obsolete. The phenotypic variance I want to assess has however also been observed in a single aphid clone before (
Preliminary work
Preliminary work during my doctoral thesis (to be submitted in 2016) focused on the constrains of the sexual polyphenism. Aphids are generally considered to benefit from climate change by extending their phenology into early spring and late autumn. During the doctoral thesis I asked whether aphids can use the novel temperature-day length combinations efficiently. I demonstrated that aphids suffer fitness constraints under short days, so the evolution of phenology might be constrained (
Project-related publications
Joschinski J, Hovestadt T, Krauss J (2015a) Coping with shorter days: do phenology shifts constrain aphid fitness? PeerJ 3: e1103. DOI: 10.7717/peerj.1103.
Joschinski J, Beer K, Helfrich-Förster C, Krauss J (2016) Pea aphids (Hemiptera: Aphididae) have diurnal rhythms when raised independently of a host plant. Journal of Insect Science 16 (31):1-5. DOI: 10.1093/jisesa/iew013.
Other publications
Joschinski J, van Kleunen, M, & Stift, M. (2015b) Costs associated with the evolution of selfing in North American populations of Arabidopsis lyrata? Evolutionary Ecology 29 (5): 749‑764. DOI: 10.1007/s10682-015-9786-3
Expected results and impact
I expect that the experiment outlined in this proposal will be the first to demonstrate a long-term fitness advantage of bet-hedging in insects. Furthermore, with the experiment I quantify the production costs and the maintenance costs of bet-hedging. I expect that the results of the experiment will be summarized in one publication (e.g. in American Naturalist or Evolution).
Depending on further collaborations, the main experiment could be supplemented by further studies. For example, I could assess the gene expression of aphids reared under the critical day length. The data could be used to study the switch in reproductive modes (photoperiodism), which would result in a further publication.
Acknowledgements
This research idea greatly benefitted from stimulating discussions within the DFG-funded collaborative research center “SFB 1047 insect timing”. I particularly thank Thomas Hovestadt, Oliver Mitesser, Christophe Gadenne and Jochen Krauss for helpful comments on the manuscript.
Funding program
Funding was provided by the German Research Foundation (DFG), collaborative research center SFB 1047 “Insect timing",
Grant title
Project C3.
Conflicts of interest
The author declares no conflict of interest.
References
-
Re-evaluating the costs and limits of adaptive phenotypic plasticity.Proceedings of the Royal Society of London. Series B: Biological Sciences277(1681):503‑511. DOI: 10.1098/rspb.2009.1355
-
Aphids on the world's crops.John Wiley & Sons,Chichester, England,476pp.
-
The pea aphid, Acyrthosiphon pisum: an emerging genomic model system for ecological, developmental and evolutionary studies.Bioessays28(7):747‑755. [Inen]. DOI: 10.1002/bies.20436
-
Adaptive phenotypic plasticity in response to climate change in a wild bird population.Science320(5877):800‑803. [Inen]. DOI: 10.1126/science.1157174
-
Adaptation, Plasticity, and Extinction in a Changing Environment: Towards a Predictive Theory.PLoS Biology8(4):20120089. DOI: 10.1371/journal.pbio.1000357
-
Optimizing reproduction in a randomly varying environment.Journal of Theoretical Biology12(1):119‑129. DOI: 10.1016/002f2-5193(66)90188-3
-
Costs and limits of phenotypic plasticity.Trends in Ecology & Evolution13(2):77‑81. [Ineng]. DOI: 10.1016/S0169-5347(97)01274-3
-
Morph determination in the bird cherry-oat aphid, Rhopalosiphum padi L.Annals of Applied Biology68(1):11‑21. [Inen]. DOI: 10.1111/j.1744-7348.1971.tb04633.x
-
Variation is function: Are single cell differences functionally important?Bioessays38(2):172‑180. DOI: 10.1002/bies.201500124
-
Dynamics of production of sexual forms in aphids: theoretical and experimental evidence for adaptive "coin-flipping" plasticity.The American Naturalist163(6):112‑25. [Ineng]. DOI: 10.1086/383618
-
Aphids as models for ecological and evolutionary studies.Insect Science21(3):247‑250. DOI: 10.1111/1744-7917.12130
-
Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change.IPCC,Geneva, Switzerland,151pp.
-
Ecology and the ratchet of events: Climate variability, niche dimensions, and species distributions.Proceedings of the National Academy of Sciences106:19685‑19692. DOI: 10.1073/pnas.0901644106
-
Coping with shorter days: do phenology shifts constrain aphid fitness?PeerJ3:e1103. [Ineng]. DOI: 10.7717/peerj.1103
-
Costs associated with the evolution of selfing in North American populations of Arabidopsis lyrata?Evolutionary Ecology29(5):749‑764. DOI: 10.1007/s10682-015-9786-3
-
Pea aphids (Hemiptera: Aphididae) have diurnal rhythms when raised independently of a host plant.Journal of Insect Science16(1):1‑5. DOI: 10.1093/jisesa/iew013
-
Climate change drives microevolution in a wild bird.Nature Communications2:208. DOI: 10.1038/ncomms1213
-
Sexual morph determination in the aphid, Acyrthosiphon pisum.Journal of Insect Physiology18(10):2029‑2042. DOI: 10.1016/0022-1910(72)90170-9
-
The role of photoperiod and temperature in the determination of parthenogenetic and sexual forms in the aphid Megoura viciae Buckton—I.Journal of Insect Physiology3(2):92‑117. DOI: 10.1016/0022-1910(59)90024-1
-
The role of photoperiod and temperature in the determination of parthogenetic and sexual forms in the aphid Megoura viciae Buckton—II. The operation of the ‘interval timer’ in young clones.Journal of Insect Physiology4(2):154‑175. DOI: 10.1016/0022-1910(60)90078-0
-
The role of photoperiod and temperature in the determination of parthenogenetic and sexual forms in the aphid Megoura viciae Buckton—III. Further properties of the maternal switching mechanism in apterous aphids.Journal of Insect Physiology9(2):153‑164. DOI: 10.1016/0022-1910(63)90067-2
-
Genome expression control during the photoperiodic response of aphids.Physiological Entomology38(2):117‑125. [InEnglish]. DOI: 10.1111/phen.12021
-
Shifting from clonal to sexual reproduction in aphids: physiological and developmental aspects.Biology of the Cell100(8):441‑451. [Inen]. DOI: 10.1042/bc20070135
-
Transcriptomic profiling of the reproductive mode switch in the pea aphid in response to natural autumnal photoperiod.Journal of Insect Physiology58(12):1517‑24. [Ineng]. DOI: 10.1016/j.jinsphys.2012.07.009
-
The evolution of aphid life cycles.Annual Review of Entomology37(1):321‑348. DOI: 10.1146/annurev.en.37.010192.001541
-
Constraints on the evolution of phenotypic plasticity: limits and costs of phenotype and plasticity.Heredity115(4):293‑301. DOI: 10.1038/hdy.2015.8
-
Ecology and evolution of sex in aphids.Trends in Ecology & Evolution17(1):34‑39. DOI: 10.1016/s0169-5347(01)02331-x
-
Modes of response to environmental change and the elusive empirical evidence for bet hedging.Proceedings of the Royal Society B: Biological Sciences278(1712):1601‑1609. DOI: 10.1098/rspb.2011.0176
-
Developmental instability as a bet-hedging strategy.Oikos80(2):401‑406. [InEnglish]. DOI: 10.2307/3546608
-
Environmental and genetic sources of diversification in the timing of seed germination: implications for the evolution of bet hedging.Evolution60(11):2280‑2292. DOI: 10.1554/05-396.1
-
Latitudinal variation in the photoperiodic responses of populations of pea aphid (Homoptera: Aphididae).Environmental Entomology19(3):618‑624. DOI: 10.1093/ee/19.3.618
-
Genome sequence of the Pea aphid Acyrthosiphon pisum.PLoS Biology8(2):e1000313. DOI: 10.1371/journal.pbio.1000313
-
Is the response of aphids to alarm pheromone stable?Journal of Applied Entomology139(10):741‑746. DOI: 10.1111/jen.12262
-
Genetic evolution, plasticity, and bet-hedging as adaptive responses to temporally autocorrelated fluctuating selection: A quantitative genetic model.Evolution69(8):2034‑2049. DOI: 10.1111/evo.12716
-
Accelerating extinction risk from climate change.Science348(6234):571‑573. DOI: 10.1126/science.aaa4984
-
Constraints on the evolution of adaptive phenotypic plasticity in plants.New Phytologist166(1):49‑60. DOI: 10.1111/j.1469-8137.2004.01296.x
-
Quantitative assessment of the importance of phenotypic plasticity in adaptation to climate change in wild bird populations.PLoS Biology11(7):1001605. DOI: 10.1371/journal.pbio.1001605
-
A second generation of homogenized Canadian monthly surface air temperature for climate trend analysis.Journal of Geophysical Research: Atmospheres117(18):D18110. DOI: 10.1029/2012JD017859
-
Mosaic physiology from developmental noise: within-organism physiological diversity as an alternative to phenotypic plasticity and phenotypic flexibility.Journal of Experimental Biology217(1):35‑45. [InEnglish]. DOI: 10.1242/jeb.089698