|
Research Ideas and Outcomes : Case Study
|
|
Corresponding author: István Mikó (istvan.miko@gmail.com)
Received: 30 Jan 2017 | Published: 31 Jan 2017
© 2017 Bipana Paudel Timilsena, István Mikó
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation: Paudel Timilsena B, Mikó I (2017) Know your insect: The structural backgrounds of regurgitation, a case study on Manduca sexta and Heliothis virescens (Lepidoptera: Sphingidae, Noctuidae). Research Ideas and Outcomes 3: e11997. https://doi.org/10.3897/rio.3.e11997
|

|
Abstract
Background
Insect herbivores often regurgitate on wounded sites while feeding on plants. Their regurgitant contains different kinds of elicitors that could trigger different plant responses. While feeding on the same host plant, Heliothis virescens and Manduca sexta caterpillars deposit different amount of regurgitant on the damage site (
New information
We compared the gross morphology of fore and midgut of the rarely regurgitating caterpillar of Manduca sexta with the more often regurgitating caterpillar of Heliothis virescens. The foregut of the rarely regurgitating caterpillar is longer than that of the regurgitating caterpillar, which contradicts the literature data.
Keywords
regurgitation, herbivory, elicitor molecules
Overview of regurgitation in insects
Insect herbivores have evolved a myriad of strategies to suppress plant anti-herbivore defensive responses. One of the herbivore strategies receiving most attention by researchers is the use of herbivore elicitors to suppress induced plant defense mechanisms. While feeding on plants, insect herbivores regurgitate on wounded sites (
Herbivores deposit different amounts of regurgitant on damaged sites of host plant (
Methodology
Heliothis virescens eggs purchased from Benzon Research (Carlisle, PA) were used to start laboratory colonies at Penn State University (State College, PA). Manduca sexta eggs were obtained from the Stephenson Lab, Penn state. Newly hatched larvae were reared with a commercial artificial diet and kept in a growth chamber maintained at 250C and 16:8-h Light:Dark condition. Early 4th instar H. virescens larvae and early 3rd instar M. sexta larvae were used in this study.
For studies with a light microscope, the larvae were dissected in a Petri dish filled with 0.1M monobasic phosphate buffer. The fat body and the tracheal system were removed carefully without damaging the alimentary canal. The cleaned up alimentary canal was analyzed and imaged under a light microscope.
For imaging with confocal laser scanning microscope (CLSM), foregut was isolated and fixed in between two coverslips spaced with a small amount of Blue-Tack (
All the works described in this paper were done as a part of “Know your insect- ENT 597”, a course offered by the Entomology Department at The Pennsylvania State University during the fall semester 2016. During this course, every student was assigned to give a mini-lecture on the morphological structure of an organ/a system of the insect they are working on followed by dissection, imaging, and discussion of the finding in the light of previous literature.
Result and discussion
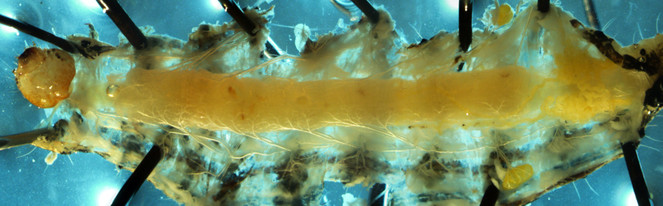
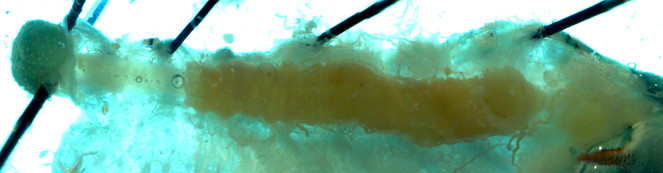
Alimentary canals of two lab reared herbivore species, M. sexta and H. virescens, were dissected under a light microscope and imaged using a confocal laser-scanning microscope (CLSM). Under the light microscope, we were able to differentiate three segments of gut (foregut, midgut and hind gut). The foregut to midgut ratio is obviously higher in Manduca sexta (Fig.
Alimentary canal of early fourth instar larvae of Heliothis virescens imaged by Light microscope
CLSM volume rendered micrograph showing the junction of foregut and midgut of Heliothis virescens larva
CLSM volume rendered micrograph showing midgut epithellium of Manduca sexta at the junction of foregut and midgut
CLSM volume rendered micrograph showing the junction of foregut and midgut of Manduca sexta larva
While feeding on the same host plant, H. virescens larvae deposit higher amounts of regurgitant than M. sexta larvae (
Two types of rhythmic movements occur in the foregut region of M. sexta larvae. Peristalsis movements push food materials towards the mid gut while constriction along the esophagus region retains food inside the crop (
Relevance to ongoing research
The senior author studies plant defense mechanisms against herbivores, mainly focusing on herbivore specificity of induced volatile production. After feeding damage by two different herbivores of the same feeding guild, Nicotiana benthamiana plant produce significantly different amounts of volatiles. Feeding damage by Heliothis virescens (generalist herbivore) produces 2–3 times as much volatiles as feeding damage by Manduca sexta (specialist herbivore). This variation is due to the presence of different elicitors in the insects’ regurgitant. M. sexta regurgitant contains volicitin, glutamine fatty acid conjugate and glutamic acid conjugates, whereas H. virescens regurgitant contains the former two elicitors only (
Acknowledgements
We thank Loren Rivera-Vega, Kirsten Persons, Ryan Reynolds, Anne Jones, Asher Jones and Samita Limbu for their help in dissections and for useful discussions during the course of this work and Missy Hazen (Penn State Microscopy and Cytometry Facility - University Park, PA) for her help with CLSM.
References
-
Differential activity and degradation of plant volatile elicitors in regurgitant of tobacco hornworm (Manduca sexta) larvae.Journal of Chemical Ecology29(6):1357‑1372. [InEnglish]. URL: http://doi.org/10.1023/A:1024209302628
-
An elicitor of plant volatiles from beet armyworm oral secretion.Science276(5314):945‑949. https://doi.org/10.1126/science.276.5314.945
-
Plant defense against insect herbivores.International Journal of Molecular Sciences14(5):10242‑10297. [InEnglish]. URL: http://doi.org/10.3390/ijms140510242
-
Diversification of gut morphology in caterpillars is associated with defensive behavior.The Journal of experimental biology209:3018‑3024. [InEnglish]. https://doi.org/10.1242/jeb.02335
-
What is fluorescing?Haemuli4(2):19‑23. URL: http://www.hymenopterists.org/newsletters/hamuli/HamuliVol4Issue2.pdf
-
The role of the frontal ganglion in foregut movements of the moth, Manduca sexta.Journal of Comparative Physiology174(6):755‑767. [InEnglish]. https://doi.org/10.1007/BF00192725
-
Do caterpillars secrete "oral secretions"?Journal of Chemical Ecology35(3):326‑335. [InEnglish]. URL: http://doi.org/10.1007/s10886-009-9604-x
-
NIH Image to ImageJ: 25 years of image analysis.Nature methods9(7):671‑675. [InEnglish]. https://doi.org/10.1038/nmeth.2089
-
Direct Proof of Ingested Food Regurgitation by Spodoptera littoralis Caterpillars during Feeding on Arabidopsis.Journal of Chemical Ecology38(7):865‑872. [InEnglish]. https://doi.org/10.1007/s10886-012-0143-5
-
N-(18-Hydroxylinolenoyl)-l-Glutamine: A Newly Discovered Analog of Volicitin in Manduca sexta and its Elicitor Activity in Plants.Journal of Chemical Ecology40(5):484‑490. [InEnglish]. URL: http://doi.org/10.1007/s10886-014-0436-y