|
Research Ideas and Outcomes : Case Study
|
|
Corresponding author: Istvan Mikó (istvan.miko@gmail.com)
Received: 16 Jan 2017 | Published: 01 Feb 2017
© 2017 Loren Rivera-Vega, Istvan Mikó
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation: Rivera-Vega L, Mikó I (2017) Know your insect: Malpighian tubules in Trichoplusia ni (Lepidoptera: Noctuidae). Research Ideas and Outcomes 3: e11827. https://doi.org/10.3897/rio.3.e11827
|

|
Abstract
Malpighian tubules are mainly known to be involved in excretion. However, recent studies have begun to look into other potential roles including detoxification, immunity, host establishment, etc. In this case study, we observed the Malpighian tubules of the cabbage looper (Trichoplusia ni) using confocal laser scanning microscopy. We also discuss other functions that Malpighian tubules are known for (i.e. silk-like and gall-inducing secretions) as well as the similarities between Malpighian tubules and salivary glands in endoparasitic Hymenoptera.
Keywords
cabbage looper, caterpillars, urinary bladder, uric acid
Case Study Background
The following work is the result of the "Know Your Insect" graduate course in the Department of Entomology at the Pennsylvania State University, taught during the fall semester of 2016. Briefly, the course provides the opportunity for a small group of students to discuss the morphology of the insects of interest. Regardless of which area of entomology we are studying (e.g. chemical ecology, physiology, agroecology, pollinators, etc.), there is invariably a structure within our model insect that plays an important role in our research. In this course, each student focuses on a structure of interest and leads the class in a lecture, discussion, and live dissection. The students also have the opportunity to image their structure of interest using confocal laser scanning microscopy (CLSM). In this particular case, we focused on the Malpighian tubules of the cabbage looper (Trichoplusia ni).
Overview of Malpighian tubules
Malpighian tubules are part of the excretory system. They are involved in osmotic and excretory regulation [
If you have ever taken an entomology course and someone mentions Malpighian tubules, the first thing that will come to mind is excretion. Or say, you have never taken an entomology course but you see the term and Google it – most hits will be about their function in excretion. Yet some other functions are also being identified.
With the explosion of high throughput techniques, the transcriptome, proteome and even metabolome of Malpighian tubules from several species are being characterized [
Malpighian tubules are also known to secret substances involved in a myriad of adaptations. For example, a common touristic destination in New Zealand are the Waitomo Glowworm Caves. The glowing that attracts so many tourists comes from Arachnocampa luminosa commonly known as the New Zealand glowworm. These are fungus gnats, which produce a bioluminescent substance in their Malpighian tubules [
Some Hemiptera from the superfamily Cercopoidea are known for producing a froth-like substance that resembles human spittle. This spittle is produced in the Malpighian tubules [
In other leafhoppers, Malpighian tubules also produce brochosomes. These are hollow proteinaceous spheres with honey-combed walls. It’s been suggested that they are used as water repellent and anti-adhesive protection against the honeydew produced by the leafhoppers while feeding [
SEM micrograph showing the brochosomes on the antenna of the putative parasitoids of Cicadellidae (Trassedia luapi, Hymenoptera, Ceraphronidae).
It has also been suggested that secretions from Malpighian tubules could be involved in host manipulation. For example, in 1914, Triggerson observed that a secretion from the Malpighian tubules in the larvae of Dryophanta erinacei was involved in the production of galls in its host [
Methodology
Rearing: Trichoplusa ni caterpillars were gathered from a laboratory colony established from eggs obtained from Benzon Research, Inc (Carlisle, PA). Larvae are kept in a growth chamber at 24oC in 16:8 Light:Dark conditions. Larvae are reared pinto bean artificial diet [
Dissections: The alimentary canal of the caterpillars were dissected out in 0.1M monobasic phosphate buffer and the Malpighian tubules along with the urinary bladders were placed in a droplet of the same buffer between two no. 1.5 coverslips with a small amount of Blue-Tack as a spacer [
Imaging: Specimens were examined with an Olympus FV10i Confocal Laser Scanning Microscope using two excitation wavelengths: 473 nm, and 559 nm. Autofluorescence was detected and assigned a pseudocolor using two channels with emission ranges of 490–590 nm (green), and 570–670 nm (red), respectively. Volume rendered micrographs and media files were created in ImageJ [
Observations on the Malpighian tubules of Trichoplusia ni using confocal laser scanning microscopy
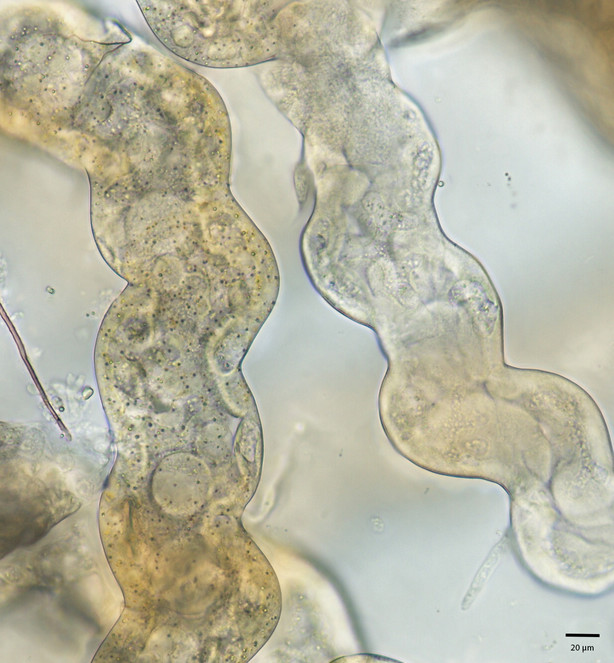
We observed the Malpighian tubules of the cabbage looper (Trichoplusia ni) using both a light microscope as well as confocal laser scanning microscope (CLSM). Under the light microscope (Fig.
Brightfield image of Malpighian tubules of Trichoplusia ni. Left: yellow region; Right: white region
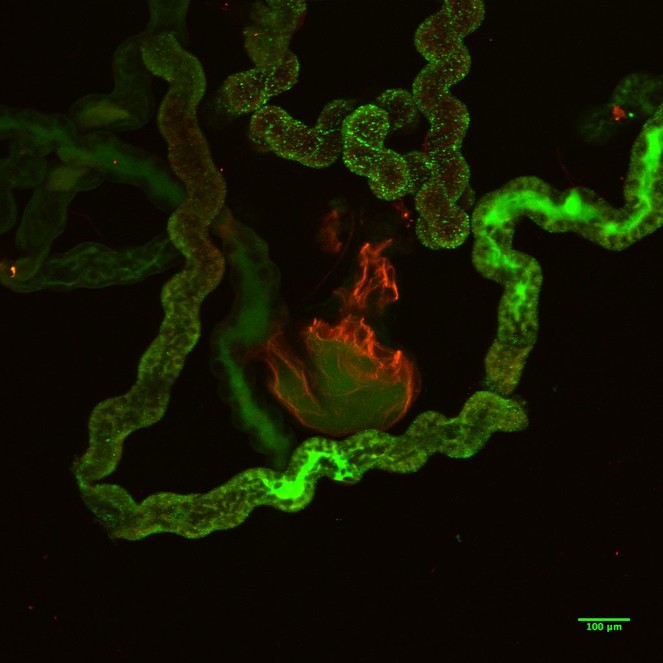
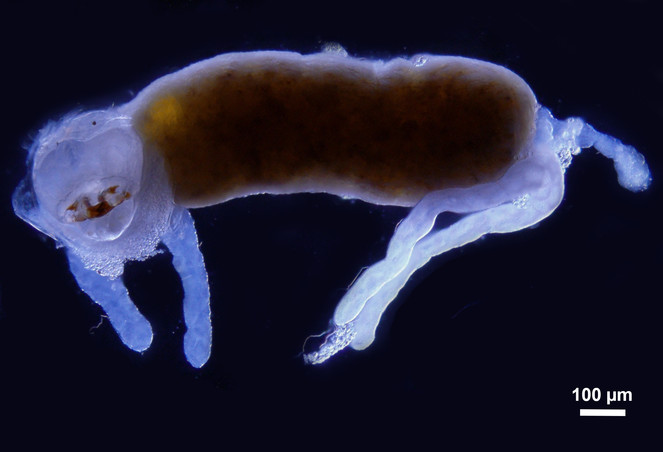
The images of the Malpighian tubules under CLSM (Figs
CLSM volume rendered micrograph showing the Malpighian tubules and the urinary bladder of Trichoplusia ni.
CLSM volume rendered micrograph showing the Malpighian tubules and the urinary bladder of Trichoplusia ni.
The reasons why cuticle tends to bioluminesce at different wavelengths from soft tissue is still not fully understood. However, this case study is an example of how useful it can be to use CLSM in order to determine potential changes in the composition of certain structures and how it can lead to the development of testable hypotheses about the function of different tissues.
Relevance to ongoing research
Ongoing research by LRV focuses on understanding the complex interactions between insects and plants.
This research specifically studies the changes that occur in the saliva of cabbage looper (Trichoplusia ni) – a generalist pest. When an insect attempts to establish on a host, there are several challenges they need to overcome. Among these, is being able to deal with the plant defenses. Not only do plants have constitutive defenses such as physical barriers (thorns, trichomes, latex, etc.) or basal levels of secondary compounds, but upon herbivory, plants will also induce defenses such as higher levels of secondary compounds or volatiles that will attract predators and/or parasitoids [
Relationship between Malpighian tubules and salivary glands
Same as with Malpighian tubules, next generation sequencing is revealing roles of saliva not previously studied such as detoxification and immunity. Malpighian tubules are also capable of secreting silk-like substances. This ‘malpighian silk’ has been observed in Neuroptera, Coleoptera and Hymenoptera [
Associations between salivary glands and MTs have been in the literature for almost 100 years. In 1938, Flanders [
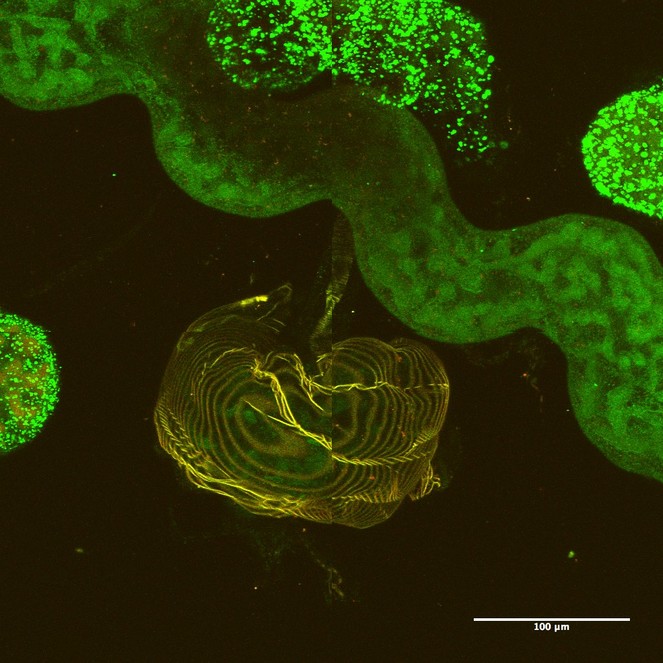
Parasitic Hymenoptera larvae contain ileac glands which are homologous to Malpighian tubules (Fig.
Alimentary canal of the second instar larva of a cynipini inquiline (Synergus sp.) showing the iliac glands (Malpighian tubule analog) and salivary glands.
All this information points to several shared functions in both salivary glands and Malpighian tubules. Could it be plants are also able to detect presence of herbivory based on secretions produced on Malpighian tubules? Or is it also possible that adaptations in Malpighian tubules are allowing generalist insects to establish on a host?
Acknowledgements
We thank Bipana Paudel, Kirsten Persons, Ryan Reynolds, Anne Jones, Asher Jones and Samita Limbu for their help in dissections and for useful discussions during the course of this work and Missy Hazen (Penn State Microscopy and Cytometry Facility - University Park, PA) for her help with CLSM.
References
-
Cues from chewing insects — the intersection of DAMPs, HAMPs, MAMPs and effectors.Current Opinion in Plant Biology26:80‑86. https://doi.org/10.1016/j.pbi.2015.05.029
-
The Developmental, Molecular, and Transport Biology of Malpighian Tubules.Annual Review of Entomology55(1):351‑374. https://doi.org/10.1146/annurev-ento-112408-085512
-
The Malpighian tubule: Rapid insights from post-genomic biology.Journal of Insect Physiology52(4):365‑378. https://doi.org/10.1016/j.jinsphys.2005.10.007
-
Larval anatomy and structure of absorbing epithelia in the aphid parasitoid Aphidius ervi Haliday (Hymenoptera, Braconidae).Arthropod Structure & Development30(1):27‑37. https://doi.org/10.1016/s1467-8039(01)00017-2
-
Transcriptomic Evidence for a Dramatic Functional Transition of the Malpighian Tubules after a Blood Meal in the Asian Tiger Mosquito Aedes albopictus.PLoS Neglected Tropical Diseases8(6):e2929. https://doi.org/10.1371/journal.pntd.0002929
-
Plant–insect dialogs: complex interactions at the plant–insect interface.Current Opinion in Plant Biology11(4):457‑463. https://doi.org/10.1016/j.pbi.2008.07.001
-
Cocoon Formation in Endoparasitic Chalcidoid.Annals of the Entomological Society of America31:167‑180. [InEnglish]. https://doi.org/10.1093/aesa/31.2.167
-
Spittle-production and tube-building by cercopid larvae (Homoptera)—IV. Mucopolysaccharide associated with spittle-production.Journal of Insect Physiology12(6):635‑644. https://doi.org/10.1016/0022-1910(66)90109-0
-
Mass-Rearing the Cabbage Looper, Trichoplusia NI, with Notes on Its Biology in the Laboratory.Annals of the Entomological Society of America53(2):229‑234. https://doi.org/10.1093/aesa/53.2.229
-
What is fluorescing?Hamuli4(2):19‑23. [InEnglish]. URL: http://www.hymenopterists.org/newsletters/hamuli/HamuliVol4Issue2.pdf
-
The rectal complex and Malpighian tubules of the cabbage looper (Trichoplusia ni): regional variations in Na+ and K+ transport and cation reabsorption by secondary cells.Journal of Experimental Biology218(20):3206‑3214. https://doi.org/10.1242/jeb.128314
-
Review: Malpighian Tubule, an Essential Organ for Insects.Entomology, Ornithology & Herpetology: Current Research03(2):1‑3. https://doi.org/10.4172/2161-0983.1000122
-
Contamination as the Cause of Erroneous Records of Brochosomes.Psyche: A Journal of Entomology2011:1‑4. https://doi.org/10.1155/2011/767963
-
Brochosomal coats turn leafhopper (Insecta, Hemiptera, Cicadellidae) integument to superhydrophobic state.Proceedings of the Royal Society B: Biological Sciences280(1752):20122391‑20122391. https://doi.org/10.1098/rspb.2012.2391
-
NIH Image to ImageJ: 25 years of image analysis.Nature Methods9:671‑675. https://doi.org/10.1038/nmeth.2089
-
A transcriptional and proteomic survey ofArachnocampa luminosa(Diptera: Keroplatidae) lanterns gives insights into the origin of bioluminescence from the Malpighian tubules in Diptera.Luminescence30(7):996‑1003. https://doi.org/10.1002/bio.2850
-
Insect Silk: One Name, Many Materials.Annual Review of Entomology55(1):171‑188. https://doi.org/10.1146/annurev-ento-112408-085401
-
A Study of Dryophanta Erinacei (Mayr) and Its Gall.Annals of the Entomological Society of America7(1):1‑34. https://doi.org/10.1093/aesa/7.1.1
-
The Myriad Plant Responses to Herbivores.Journal of Plant Regulation19(2):195‑216. https://doi.org/10.1007/s003440000026
-
Proteomic-Based Insight into Malpighian Tubules of Silkworm Bombyx mori.PLoS ONE8(9):e75731. https://doi.org/10.1371/journal.pone.0075731
Supplementary materials
Download file (28.19 MB)
Download file (8.36 MB)
Download file (8.36 MB)